Claudin-11/OSP-based tight junctions of myelin sheaths in brain and Sertoli cells in testis - PubMed (original) (raw)
Claudin-11/OSP-based tight junctions of myelin sheaths in brain and Sertoli cells in testis
K Morita et al. J Cell Biol. 1999.
Abstract
Members of the newly identified claudin gene family constitute tight junction (TJ) strands, which play a pivotal role in compartmentalization in multicellular organisms. We identified oligodendrocyte-specific protein (OSP) as claudin-11, a new claudin family member, due to its sequence similarity to claudins as well as its ability to form TJ strands in transfected fibroblasts. Claudin-11/OSP mRNA was expressed in the brain and testis. Immunofluorescence microscopy with anti-claudin-11/OSP polyclonal antibody (pAb) and anti-neurofilament mAb revealed that in the brain claudin-11/OSP-positive linear structures run in a gentle spiral around neurofilament-positive axons. At the electron microscopic level, these linear structures were identified as the so-called interlamellar strands in myelin sheaths of oligodendrocytes. In testis, well-developed TJ strands of Sertoli cells were specifically labeled with anti-claudin-11/OSP pAb both at immunofluorescence and electron microscopic levels. These findings indicated that the interlamellar strands of oligodendrocyte myelin sheaths can be regarded as a variant of TJ strands found in many other epithelial cells, and that these strands share a specific claudin species, claudin-11/OSP, with those in Sertoli cells to create and maintain the repeated compartments around axons by oligodendrocytes.
Figures
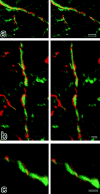
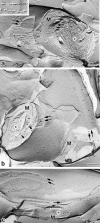
Figure 5
Stereoscopic comparison of subcellular distribution between claudin-11/OSP and neurofilaments. Frozen sections of the brain cortex were doubly stained with anti–claudin-11/OSP pAb (red) and anti-neurofilament mAb (green), examined by confocal microscopy, and stereoscopic images were generated. Note that each claudin-11/OSP-positive linear structure (red) ran in a gentle spiral around a neurofilament-positive axon (green). Bars: (a) 2 μm; (b) 1 μm; (c) 1 μm.
Figure 1
Comparison of amino acid sequences of mouse OSP and claudin-1 by the GENETYX program. Identity and homology are indicated by | and :, respectively. Four putative transmembrane domains are indicated by boxes. They showed 31.7% identity at the amino acid sequence level. Note that identical residues are distributed almost evenly through the molecule, and that OSP and claudin-1 end in -H-V and -Y-V, respectively.
Figure 2
L transfectants expressing FLAG-tagged OSP. (a–d) Immunofluorescence (a) and corresponding phase contrast images (b) of stable L transfectants expressing FLAG-OSP. Cells were stained with anti-FLAG mAb. Expressed FLAG-OSP was concentrated at cell–cell borders as planes (arrow) or on thin cellular protrusions (arrowhead). At higher magnification (c and d), at cell–cell contact planes, FLAG-OSP was concentrated as networks or as thick lines. (e) Freeze-fracture images of cell–cell contact planes of stable L transfectants expressing FLAG-OSP. At low magnification, large numbers of TJ strand/ groove-like structures were observed. These strands scarcely branched, and showed a tendency to run parallel to each other. Inset, higher magnification of strands on P-face (top) and grooves on E-face (bottom). Bars: (a and b) 10 μm; (c and d) 4 μm; (e) 500 nm; (inset) 100 nm.
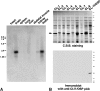
Figure 3
Northern blots of claudin-11/OSP expression and specificity of anti–claudin-11/OSP pAb. (A) Mouse Multiple Tissue Northern Blot (CLONTECH) was probed with a DNA fragment of mouse claudin-11/OSP. Claudin-11/OSP mRNA was detected as a 2.3-kb band in large amounts in the brain and testis and in only a trace amount in the kidney. (B) Immunoblots of total lysates of E. coli expressing GST fusion proteins with cytoplasmic domains of claudin-1 to -8 and claudin-11/OSP (arrow) confirmed the specificity of anti–claudin-11/OSP pAb. Bars indicate molecular masses of 200, 116, 97, 66, 45, 31, 21, 15, and 10 kD from the top.
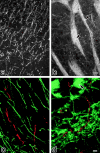
Figure 4
Distribution of claudin-11/OSP in mouse brain. Frozen sections of mouse brain, cortex region (a and c), and deeper region (b and d), were singly stained with anti–claudin-11/OSP pAb (a and b) or doubly stained with anti–claudin-11/OSP pAb (green in c and d) and anti-occludin mAb (red in c and d). In the cortex region (a and c), a large number of intensely stained linear structures was seen scattered in random directions, whereas in the deeper region (b and d) these claudin-11/OSP-positive structures were occasionally arranged in a parallel manner to form thick bundles (arrows). These claudin-11/OSP-positive linear structures did not overlap with occludin-positive endothelial TJs (c and d). Bars: (a and b) 10 μm; (c and d) 5 μm.
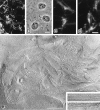
Figure 6
Localization of claudin-11/OSP at interlamellar strands in oligodendrocytes. (a) Mouse brain was fixed with glutaraldehyde and then processed for conventional freeze-fracture analysis. So-called interlamellar strands (arrows) were observed in each lamella of myelin sheaths. Asterisk, transversely fractured image of axoplasm; M, transversely fractured image of myelin sheaths. Inset, enlarged image of interlamellar strands. (b and c) Mouse brain (b) or optic nerve (c) was quickly frozen without chemical fixation, and then processed for freeze-fracture analysis. Freeze-fracture replicas were labeled with anti–claudin-11/OSP pAb. Interlamellar strands (arrows) were specifically labeled with the pAb (10-nm gold particles). Note that the transversely fractured myelin sheaths were also labeled with the pAb (arrowheads), and that this labeling pattern was very similar to Fig. 7. Asterisk, transversely fractured image of axoplasm; M, transversely fractured image of myelin sheaths. Bars: (a) 100 nm; (b and c) 200 nm.
Figure 7
Localization of claudin-11/OSP at radial components in myelin sheaths. (a and b) Ultrathin cryo-sections of the mouse brain were labeled with anti–claudin-11/ OSP pAb. Note that transverse sections of myelin sheaths were specifically labeled radially (10-nm gold particles; arrows). Asterisk, transverse section of axoplasm; M, transverse section of myelin sheaths. Bar, 100 nm.
Figure 8
Subcellular localization of claudin-11/OSP at TJ strands in Sertoli cells. (a and b) Frozen sections of mouse testis were doubly stained with anti–claudin-11/ OSP pAb (a) and anti-occludin mAb (b). Both claudin-11/OSP and occludin were concentrated and precisely colocalized in a linear fashion at the most basal region of lateral membranes of adjacent Sertoli cells (arrowheads). Note that vascular endothelial cells were stained positively for occludin but were negative for claudin-11/ OSP (arrow). Asterisks, centers of seminiferous tubules. (c) Mouse testes were quickly frozen without chemical fixation, and then processed for freeze-fracture. Freeze-fracture replicas were labeled with anti–claudin-11/OSP pAb. Characteristic Sertoli TJs were exclusively labeled with the pAb (10-nm gold particles). Bars: (a and b) 25 μm; (c) 200 nm.
Similar articles
- CNS myelin and sertoli cell tight junction strands are absent in Osp/claudin-11 null mice.
Gow A, Southwood CM, Li JS, Pariali M, Riordan GP, Brodie SE, Danias J, Bronstein JM, Kachar B, Lazzarini RA. Gow A, et al. Cell. 1999 Dec 10;99(6):649-59. doi: 10.1016/s0092-8674(00)81553-6. Cell. 1999. PMID: 10612400 - OSP/claudin-11 forms a complex with a novel member of the tetraspanin super family and beta1 integrin and regulates proliferation and migration of oligodendrocytes.
Tiwari-Woodruff SK, Buznikov AG, Vu TQ, Micevych PE, Chen K, Kornblum HI, Bronstein JM. Tiwari-Woodruff SK, et al. J Cell Biol. 2001 Apr 16;153(2):295-305. doi: 10.1083/jcb.153.2.295. J Cell Biol. 2001. PMID: 11309411 Free PMC article. - A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts.
Furuse M, Sasaki H, Fujimoto K, Tsukita S. Furuse M, et al. J Cell Biol. 1998 Oct 19;143(2):391-401. doi: 10.1083/jcb.143.2.391. J Cell Biol. 1998. PMID: 9786950 Free PMC article. - Involvement of OSP/claudin-11 in oligodendrocyte membrane interactions: role in biology and disease.
Bronstein JM, Tiwari-Woodruff S, Buznikov AG, Stevens DB. Bronstein JM, et al. J Neurosci Res. 2000 Mar 15;59(6):706-11. doi: 10.1002/(SICI)1097-4547(20000315)59:6<706::AID-JNR2>3.0.CO;2-D. J Neurosci Res. 2000. PMID: 10700007 Review. - The structure and function of claudins, cell adhesion molecules at tight junctions.
Tsukita S, Furuse M. Tsukita S, et al. Ann N Y Acad Sci. 2000;915:129-35. doi: 10.1111/j.1749-6632.2000.tb05235.x. Ann N Y Acad Sci. 2000. PMID: 11193568 Review.
Cited by
- Pores in the wall: claudins constitute tight junction strands containing aqueous pores.
Tsukita S, Furuse M. Tsukita S, et al. J Cell Biol. 2000 Apr 3;149(1):13-6. doi: 10.1083/jcb.149.1.13. J Cell Biol. 2000. PMID: 10747082 Free PMC article. Review. No abstract available. - The structure and function of myelin: from inert membrane to perfusion pump.
Dyer CA. Dyer CA. Neurochem Res. 2002 Nov;27(11):1279-92. doi: 10.1023/a:1021611430052. Neurochem Res. 2002. PMID: 12512934 Review. - Diabetes, insulin-mediated glucose metabolism and Sertoli/blood-testis barrier function.
Alves MG, Martins AD, Cavaco JE, Socorro S, Oliveira PF. Alves MG, et al. Tissue Barriers. 2013 Apr 1;1(2):e23992. doi: 10.4161/tisb.23992. Tissue Barriers. 2013. PMID: 24665384 Free PMC article. Review. - Opalin, a transmembrane sialylglycoprotein located in the central nervous system myelin paranodal loop membrane.
Yoshikawa F, Sato Y, Tohyama K, Akagi T, Hashikawa T, Nagakura-Takagi Y, Sekine Y, Morita N, Baba H, Suzuki Y, Sugano S, Sato A, Furuichi T. Yoshikawa F, et al. J Biol Chem. 2008 Jul 25;283(30):20830-40. doi: 10.1074/jbc.M801314200. Epub 2008 May 19. J Biol Chem. 2008. PMID: 18490449 Free PMC article. - Claudin Proteins And Neuronal Function.
Devaux J, Fykkolodziej B, Gow A. Devaux J, et al. Curr Top Membr. 2010;65:229-253. doi: 10.1016/S1063-5823(10)65010-7. Curr Top Membr. 2010. PMID: 25013353 Free PMC article.
References
- Anderson JM, Van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol. 1995;269:G467–G475. - PubMed
- Balda MS, Anderson JM. Two classes of tight junctions are revealed by ZO-1 isoforms. Am J Physiol. 1993;264:C918–C924. - PubMed
- Balda MS, Whitney JA, Flores C, González S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996;134:1031–1049. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases