The secretory route of the leaderless protein interleukin 1beta involves exocytosis of endolysosome-related vesicles - PubMed (original) (raw)
The secretory route of the leaderless protein interleukin 1beta involves exocytosis of endolysosome-related vesicles
C Andrei et al. Mol Biol Cell. 1999 May.
Free PMC article
Abstract
Interleukin 1beta (IL-1beta), a secretory protein lacking a signal peptide, does not follow the classical endoplasmic reticulum-to-Golgi pathway of secretion. Here we provide the evidence for a "leaderless" secretory route that uses regulated exocytosis of preterminal endocytic vesicles to transport cytosolic IL-1beta out of the cell. Indeed, although most of the IL-1beta precursor (proIL-1beta) localizes in the cytosol of activated human monocytes, a fraction is contained within vesicles that cofractionate with late endosomes and early lysosomes on Percoll density gradients and display ultrastructural features and markers typical of these organelles. The observation of organelles positive for both IL-1beta and the endolysosomal hydrolase cathepsin D or for both IL-1beta and the lysosomal marker Lamp-1 further suggests that they belong to the preterminal endocytic compartment. In addition, similarly to lysosomal hydrolases, secretion of IL-1beta is induced by acidotropic drugs. Treatment of monocytes with the sulfonylurea glibenclamide inhibits both IL-1beta secretion and vesicular accumulation, suggesting that this drug prevents the translocation of proIL-1beta from the cytosol into the vesicles. A high concentration of extracellular ATP and hypotonic medium increase secretion of IL-1beta but deplete the vesicular proIL-1beta content, indicating that exocytosis of proIL-1beta-containing vesicles is regulated by ATP and osmotic conditions.
Figures
Figure 1
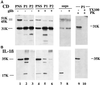
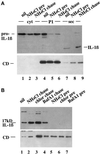
ProIL-1β is contained in part in vesicles cofractionating with lysosomes. Western blot analysis of PNS (5% of total, lanes 1 and 4), P1 (lanes 2 and 5), and P2 (lanes 3 and 6) or supernatants from 106 cells (lanes 7 and 8) obtained from activated monocytes cultured in the absence (−) or presence (+) of 100 μM glibenclamide (glib). After removal of 5% PNS, PNS were treated with proteinase K (PK) before the ultracentrifugation. Lanes 9 and 10, P1 from undigested PNS was untreated (lane 9) or solubilized (+TX100, lane 10) with Triton X-100 before proteinase K (PK) digestion. Filters were hybridized with rabbit anti-CD (A), melted, and rehybridized with mouse anti-IL-1β (B) antibodies. The migration of the three molecular forms of CD and proIL-1β and IL-1β is indicated.
Figure 2
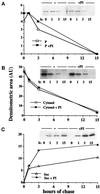
Kinetic analyses of cytosolic and particulated IL-1β. LPS-activated monocytes were pulsed with 1 mCi/ml methionine-cysteine Promix [35S] for 15 min (time of chase, 0) and chased in complete medium for 1, 3, 6, or 15 h in the absence (open symbols) or presence (closed symbols) of 100 μg of pepstatin and leupeptin. At the end of each period of chase subcellular fractionation was carried out as above, P1 and P2 were treated with proteinase K, followed by protease inhibitors and solubilization, and the IL-1β present in the pooled P1 and P2 fractions (A), in the cytosol (B), and in the supernatants (C) was analyzed by immunoprecipitation with anti-IL-1β antiserum followed by SDS-PAGE and autoradiography as described (Rubartelli et al., 1990). Data are expressed as densitometric areas (AU, arbitrary units). One representative experiment of three performed is shown.
Figure 3
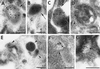
Immunoelectron microscopy analysis of the colocalization of IL-1β and CD (A–C, E, and F) and of IL1β and Lamp-1 (D and G) in P1 fraction. Double immunolabeling with anti-IL-1β (10-nm gold particles) and anti-CD (18-nm gold particles) antibodies reveals the presence of both molecules in organelles (>200 nm in diameter), which display the typical morphology of late endosomes and early lysosomes (A, arrow) or more dense, mature lysosomes (B, arrow). These organelles appear double immunolabeled also for IL-1β (10-nm gold particles) and Lamp-1 (18-nm gold particles) (D, arrow). Mature lysosomes showing positive staining for IL-1β alone are also found (C, arrow). Dense vesicles, <200 nm in diameter, appear either positively immunolabeled for IL-1β only (E, arrowheads), for both IL-1β and CD (F, arrowhead), or for both IL-1β and Lamp-1 (G, arrowhead). Bars, 200 nm.
Figure 4
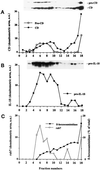
Migration of proIL-1β on Percoll density gradient. P1 and P2 fractions were pooled and further fractionated by centrifugation on a 25% Percoll density gradient under conditions that separate heavy-density lysosomes from lower-density endosomes. Membranes were collected from the individual fractions and tested for β-hexosoaminidase activity or analyzed by Western blot using anti-CD, anti-IL-1β, or anti-Rab7 antibodies. Fraction 1 represents the lowest density; fraction 18 is the highest density. The reactivity of proCD and CD (A), IL-1β (B), and Rab7 (C) was quantified by image digitalization and plotted as arbitrary units (a.u.); β-hexosoaminidase activity (C) is expressed as percent of total. The Western blots hybridized with anti-CD and anti-IL-1β are shown as insets in A and B, respectively. One representative experiment four is shown.
Figure 5
Effects of lysosomotropic drug treatment on secretion and vesicular accumulation of IL-1β. A, Monocytes were stimulated 1 h with LPS without (nil; lanes 1, 4, and 7) or with 50 mM NH4Cl (NH4Cl pre; lanes 2, 5, and 8) and cultured 2.5 h in the absence or presence of the same drug as indicated. Alternatively, monocytes were stimulated 1 h with LPS without NH4Cl, followed by 2.5 h of culture with the drug (NH4Cl chase; lanes 3, 6, and 9). Filters were hybridized with anti-IL-1β (upper panels) or anti-CD (lower panels) antibodies. Cyt, cytosolic fractions (lanes 1–3); P1, P1 pellet (lanes 4–6); sec, supernatants (lanes 7–9). (B) Supernatants from monocytes untreated (nil, lane 1), chased (lanes 2–4), or pretreated (lanes 5–7) as in A with 50 mM NH4Cl (lanes 2 and 5), 50 μM chloroquine (chlor; lanes 3 and 6), or 1 μM BafA1 (lanes 4 and 7). Filters were hybridized with anti-IL-1β antibody.
Figure 6
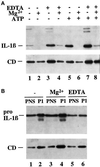
Exocytosis of IL-1β–containing vesicles is regulated by extracellular [Mg2+]. (A) Supernatants from monocytes incubated 2.5 h with LPS in medium alone (lanes 1 and 5) or plus 5 mM EDTA (lanes 3 and 7), 5 mM MgCl2 (lanes 2 and 6), or 5 mM EDTA plus 5 mM MgCl2 (lanes 4 and 8). When indicated (+ATP, lanes 5–8), 1 mM ATP was added during the last 30 min. (B) PNS (5%) and P1 from activated monocytes cultured 1 h in the absence (−, lanes 1 and 2) or presence of 5 mM MgCl2 (Mg2+, lanes 3 and 4) or 5 mM EDTA (lanes 5 and 6). Filters were hybridized with anti-IL-1β (upper panels) or anti-CD (lower panels).
Figure 7
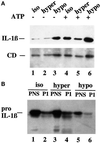
Exocytosis of IL-1β–containing vesicles is regulated by extracellular osmotic conditions. (A) Supernatants from monocytes incubated 2.5 h with LPS in isotonic conditions (medium alone, iso, lanes 1 and 4) or plus 0.1 M sucrose (hyper, lanes 2 and 5) or diluted 1:2 with water (hypo, lanes 3 and 6) in the absence (−, lanes 1–3) or presence of 1 mM ATP (+, lanes 4–6) for the last 30 min. Filters were hybridized with anti-IL-1β (upper panels) or anti-CD (lower panels). (B) PNS and P1 from monocytes incubated 2.5 h with LPS in isotonic conditions (medium alone, iso, lanes 1 and 2) or plus 0.1 M sucrose (hyper, lanes 3 and 4) or diluted 1:2 with water (hypo, lanes 5 and 6).
Figure 8
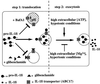
Two step model for IL-1β secretion, showing vesicle-mediated transport from the cytosol to the extracellular space. The translocation step is blocked by glibenclamide and when the ΔpH between cytosol and vesicles is abolished; the exocytosis is induced by high extracellular ATP and by hypotonic medium, whereas it is inhibited by low extracellular ATP and by hypertonic conditions.
Similar articles
- Interleukin-1beta secretion is impaired by inhibitors of the Atp binding cassette transporter, ABC1.
Hamon Y, Luciani MF, Becq F, Verrier B, Rubartelli A, Chimini G. Hamon Y, et al. Blood. 1997 Oct 15;90(8):2911-5. Blood. 1997. PMID: 9376570 - P2X7 receptor regulation of non-classical secretion from immune effector cells.
Dubyak GR. Dubyak GR. Cell Microbiol. 2012 Nov;14(11):1697-706. doi: 10.1111/cmi.12001. Epub 2012 Aug 24. Cell Microbiol. 2012. PMID: 22882764 Free PMC article. Review. - Progressive waves of IL-1β release by primary human monocytes via sequential activation of vesicular and gasdermin D-mediated secretory pathways.
Semino C, Carta S, Gattorno M, Sitia R, Rubartelli A. Semino C, et al. Cell Death Dis. 2018 Oct 23;9(11):1088. doi: 10.1038/s41419-018-1121-9. Cell Death Dis. 2018. PMID: 30352992 Free PMC article. - Human monocyte interleukin-1beta posttranslational processing. Evidence of a volume-regulated response.
Perregaux DG, Laliberte RE, Gabel CA. Perregaux DG, et al. J Biol Chem. 1996 Nov 22;271(47):29830-8. doi: 10.1074/jbc.271.47.29830. J Biol Chem. 1996. PMID: 8939922 - Evolution, role in inflammation, and redox control of leaderless secretory proteins.
Sitia R, Rubartelli A. Sitia R, et al. J Biol Chem. 2020 May 29;295(22):7799-7811. doi: 10.1074/jbc.REV119.008907. Epub 2020 Apr 24. J Biol Chem. 2020. PMID: 32332096 Free PMC article. Review.
Cited by
- Genetic loss of murine pyrin, the Familial Mediterranean Fever protein, increases interleukin-1β levels.
Hesker PR, Nguyen M, Kovarova M, Ting JP, Koller BH. Hesker PR, et al. PLoS One. 2012;7(11):e51105. doi: 10.1371/journal.pone.0051105. Epub 2012 Nov 30. PLoS One. 2012. PMID: 23226472 Free PMC article. - The endolysosomal system in conventional and unconventional protein secretion.
Néel E, Chiritoiu-Butnaru M, Fargues W, Denus M, Colladant M, Filaquier A, Stewart SE, Lehmann S, Zurzolo C, Rubinsztein DC, Marin P, Parmentier ML, Villeneuve J. Néel E, et al. J Cell Biol. 2024 Sep 2;223(9):e202404152. doi: 10.1083/jcb.202404152. Epub 2024 Aug 12. J Cell Biol. 2024. PMID: 39133205 Free PMC article. Review. - Maedi-visna virus and caprine arthritis encephalitis virus genomes encode a Vpr-like but no Tat protein.
Villet S, Bouzar BA, Morin T, Verdier G, Legras C, Chebloune Y. Villet S, et al. J Virol. 2003 Sep;77(17):9632-8. doi: 10.1128/jvi.77.17.9632-9638.2003. J Virol. 2003. PMID: 12915575 Free PMC article. - P2X(7) receptor at the heart of disease.
Vasileiou E, Montero RM, Turner CM, Vergoulas G. Vasileiou E, et al. Hippokratia. 2010 Jul;14(3):155-63. Hippokratia. 2010. PMID: 20981163 Free PMC article. - Histone deacetylase inhibitors prevent exocytosis of interleukin-1beta-containing secretory lysosomes: role of microtubules.
Carta S, Tassi S, Semino C, Fossati G, Mascagni P, Dinarello CA, Rubartelli A. Carta S, et al. Blood. 2006 Sep 1;108(5):1618-26. doi: 10.1182/blood-2006-03-014126. Epub 2006 May 9. Blood. 2006. PMID: 16684958 Free PMC article.
References
- Ayala JM, Yamin TT, Egger LA, Chin J, Kostura MJ, Miller DE. IL-1β-converting enzyme is present in monocytic cells as inactive precursor. J Immunol. 1994;153:2592–2599. - PubMed
- Becq F, Hamon Y, Bajetto A, Gola M, Verrier B, Chimini G. ABC1, an ATP binding cassette transporter, required during apoptosis, generates a regulated ion flux after expression in Xenopus oocytes. J Biol Chem. 1997;272:2695–2701. - PubMed
- Borregaard N, Lollike K, Kjeldsen L, Sengelov H, Bastholm L, Nielsen MH, Baiton DF. Human neutrophil granules and secretory vesicles. Eur J Hematol. 1993;51:187–198. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous