Fibronectin regulates assembly of actin filaments and focal contacts in cultured cells via the heparin-binding site in repeat III13 - PubMed (original) (raw)
Fibronectin regulates assembly of actin filaments and focal contacts in cultured cells via the heparin-binding site in repeat III13
L Bloom et al. Mol Biol Cell. 1999 May.
Free PMC article
Abstract
Fibroblasts, when plated on the extracellular matrix protein fibronectin (FN), rapidly spread and form an organized actin cytoskeleton. This process is known to involve both the central alpha5beta1 integrin-binding and the C-terminal heparin-binding regions of FN. We found that within the heparin-binding region, the information necessary for inducing organization of stress fibers and focal contacts was located in a 29-amino acid segment of FN type III module 13 (III13). We did not find a cytoskeleton-organizing role for repeat III14, which had previously been implicated in this process. Within III13, the same five basic amino acids known to be most important for heparin binding were also necessary for actin organization. A substrate of III13 alone was only weakly adhesive but strongly induced formation of filopodia and lamellipodia. Stress fiber formation required a combination of III13 and III7-11 (which contains the integrin alpha5beta1 recognition site), either as a single fusion protein or as separate polypeptides, and the relative amounts of the two binding sites appeared to determine whether stress fibers or filopodia and lamellipodia were the predominant actin structures formed. We propose that a balance of signals from III13 and from integrins regulates the type of actin structures assembled by the cell.
Figures
Figure 1
GST–His6 vectors and FN fusion proteins. (A) Polycloning regions of the vectors pGH and pGH.PL. DNA sequences, shown grouped into codons, begin after the last codon of the GST coding region and end at the TAA stop codon that formed part of the _Eco_RI site of pGEX-2T. Restriction sites and the sequence encoding the His6 tag are indicated. (B) Schematic drawing of FN and recombinant GST-FN-His6 fusion proteins. Small rectangles, FN type I repeats; open circles, FN type II repeats; squares, FN type III repeats. Important FN sites are denoted by filled symbols: circles, RGD sequence; arrowhead pointing up, heparin-binding site; arrowhead pointing down, PRARI; shaded area, V region.
Figure 2
Actin organization in MG-63 cells on FN and recombinant fragments in the presence or absence of heparin. Cells were plated for 2 h on 20 μg/ml FN (A and D), 10 μg/ml 120-kDa fragment of FN (B and E), which contains the central cell-binding domain but not the heparin-binding domain, or 10 μg/ml F7–15 (C and F), a GST–FN–His6 fusion containing both the cell-binding and heparin-binding domains. Rhodamine–phalloidin-stained cells without heparin (A–C) or with 100 μg/ml heparin (D–F) applied to substrates before plating and to cells during incubation. Cells in A and C are representative of cells with well-organized stress fibers in quantitative stress fiber assays. Bar, 10 μm.
Figure 3
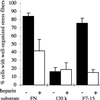
Heparin inhibits stress fiber formation in MG-63 cells on FN or a recombinant FN fragment. Cells were plated for 2 h on FN, 120-kDa fragment, or F7–15 as described in Figure 2. The percentage of cells with well-organized stress fibers (see Figure 2, A and C) was scored (mean ± SD for duplicate samples).
Figure 4
Repeat III13, together with repeats III7–11, supports full stress fiber and focal contact formation. Cells were plated for 2 h on a 7.5 μg/ml concentration of each substrate and then fixed and stained for vinculin (A–J) and actin (A′–J′). (A and A′) F7–15; (B and B′) F7–11; (C and C′) F7–11,10; (D and D′) F7–11, EIIIB; (E and E′) F7–11,12; (F and F′) F7–11,13; (G and G′) F7–11,14; (H and H′) F7–11,13–14; (I and I′) F7–15(14SS); (J and J′) F7–11,13(R7S/K25S) (see text below). Arrowheads in A, representative focal contacts at cell apices; arrows in A, representative internal focal contacts; arrows in B, representative peripheral vinculin patches in cells with poor actin organization. Cells in A, F, H, and I are representative of cells scored as having well-organized focal contacts. Bar, 10 μm.
Figure 5
III13 is unique in its stress fiber– and focal contact–inducing ability and is as potent as the entire heparin-binding domain. MG-63 cells were plated on the GST–F7–11, type III–His6 fusions for 2 h, and the proportions of cells with well-organized stress fibers and vinculin-containing focal contacts were scored (mean ± SD for duplicate samples). (A) Stress fiber– and focal contact–organizing activities of F7–11,13 but not other F7–11, type III repeat fusions, are similar to those of F7–15. Cells were plated on 7.5 μg/ml fusion protein for 2 h and scored for distribution of vinculin (black bars) and actin (open bars). (B) Dose dependence of stress fiber–organizing activity of F7–11, type III repeat fusions. 7–11,13 (▪), 7–11,12 (□), 7–11,B (▴), 7–11,10 (○), and 7–11,14 (●) were tested for stress fiber–inducing activity over a range of concentrations. (C) Mutation of the PRARI site in III14 does not alter stress fiber induction by F7–15. F7–15 (▪), F7–15(14SS) (□), and F7–11,13 (▵) were tested for stress fiber–organizing activity over a range of concentrations.
Figure 6
The N-terminal 29 amino acids of repeat III13 confer stress fiber–inducing activity on repeat III12. (A) Schematic drawing of repeat III13, based on the crystal structure of repeat III10 (Dickinson et al., 1994) and confirmed by the recently solved crystal structure of the heparin-binding domain (Sharma et al., 1999). Beta strands A–G are shown below the domain. The heparin-binding amino acids R6, R7, R9, R23, K25, and R54 are circled (Busby et al., 1995). Dashed lines show the parts of III13 used in III12/III13 chimeras, designated AB + loop and DE. (B) Schematic drawing of III12/III13 chimeras, showing regions of III13 in black and III12 in white. Beta strands are drawn above. The percentages of cells with organized stress fibers when plated on F7–11, III12/III13 chimeras at 9 μg/ml (mean ± SD) are shown at right.
Figure 7
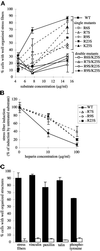
Heparin-binding site mutations reduce stress fiber– and focal contact–inducing activity of III13 and confer partial resistance to heparin. (A) Effects of heparin-binding site mutations on stress fiber formation. MG-63 cells were plated for 2 h on wild-type and mutant F7–11,13 substrates over a range of concentrations, and stress fiber formation was scored (mean ± SD of duplicate samples). ▪, wild-type F7–11,13. Single mutants are indicated by dashed lines: □, R6S; ▵, R7S; ○, R9S; ▪, R23S; ▴, K25S. Double mutants are indicated by solid lines: □, R6S/K25S; ▵, R7S/K25S; ○, R9S/R23S; ●, R9S/K25S. (B). Effects of heparin on stress fiber formation. MG-63 cells were plated for 2 h on 15 μg/ml F7–11,13 fusions carrying wild-type (▪), R7S (▵), R9S (○), or R23S (▪) mutant III13.Substrates were pretreated with 0, 10, or 100 μg/ml heparin, which remained present throughout the experiment. For each substrate, stress fiber induction is shown normalized to induction in the absence of heparin. (C) The R7S/K25S mutation in III13 reduces focal contact organization. MG63 cells plated for 2 h on F7–11,13 (black bars) or F7–11,13(R7S/K25S) (open bars) were fixed and stained for localization of actin, vinculin, paxillin, talin, and phosphotyrosine, and the percentage of cells (±SD) with well-organized focal contacts containing each protein was scored.
Figure 8
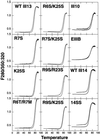
Thermal stability of type III repeats lacking stress fiber–inducing activity is normal. Purified mutant III13–His6 and III14–His6 domains showed melting profiles similar to that of their wild-type counterparts, indicating that they are properly folded. Melting properties of purified type III–His6 fusion proteins were determined by heating samples at 1°C/min and measuring the ratio of fluorescence intensity at 350 nm to that at 320 nm with excitation at 280 nm. The upper curve for each sample was obtained during cooling.
Figure 9
Differences in stress fiber–inducing activity between wild-type and mutant III13 domains do not result from differences in adhesivity. Cells adhering to varying concentrations of F7–11,13 (▪), F7–11,13(R7S/K25S) (□), F13 (▴) or F13(R7S/K25S) (▵) on glass coverslips for 15 min were fixed, stained with DAPI, and counted. Stress fiber formation was scored in a second set of cells adhering for 2 h to glass coverslips coated with F7–11,13 (●) or F7–11,13(R7S/K25S) (○).
Figure 10
Time course of actin assembly of MG-63 cells plated on 7.5 μg/ml F7–11,13 or F7–11,13(R7S/K25S). Cells were fixed at the times indicated and stained with TRITC-phalloidin. Long arrows, representative filopodia; arrowheads, representative lamellipodia extending parallel to the substratum; short arrows, representative ruffles perpendicular to the substratum. Cells shown for wild-type F7–11,13 at 2–30 min are representative of cells scored as having abundant filopodia in quantitative assays. Cells shown for F7–11,13 at 2–15 min are representative of cells with abundant lamellipodia. Bar, 10 μm.
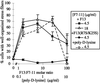
Figure 11
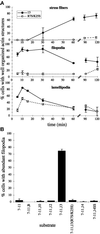
Wild-type III13 function is necessary during early and late events of actin assembly. (A) Cells were plated on 7.5 μg/ml F7–11,13 or F7–11,13(R7S/K25S) and fixed at intervals and stained with TRITC-phalloidin. The percentages of cells with well-organized stress fibers, abundant filopodia (>10 per cell), or abundant lamellipodia (>25% of the cell perimeter occupied by actin-rich sheets) were scored (±SD, in duplicate samples). ●, F7–11,13; □, F7–11,13(R7S/K25S). (B) Formation of filopodia on recombinant FN fragments. The percentages of cells with abundant filopodia were scored in cells plated for 15 min on 15 μg/ml F7–11,type III repeat substrates.
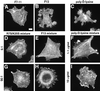
Figure 12
GST–III13–His6 or poly-
d
-lysine alone can support cell adhesion and extension of filopodia and lamellipodia and can induce stress fibers in a mixed substrate with F7–11. Cells were stained with TRITC-phalloidin after 2 h on 4.5 μg/ml F7–11 alone (A), 206 μg/ml GST–III13–His6 alone (B), 1.1 μg/ml poly-
d
-lysine alone (C), or the following mixed substrates: 4.5 μg/ml F7–11 together with mutant GST–III13(R7SK25S)–His6 at 12.9 μg/ml (D; molar ratio, 6 GST–III13(R7SK25S)–His6:1 F7–11) or 206 μg/ml (G; 96:1 molar ratio), 4.5 μg/ml F7–11 with wild-type GST–III13–His6 at 12.9 μg/ml (E; 6:1) or 206 μg/ml (H; 96:1), or 4.5 μg/ml F7–11 together with poly-
d
-lysine at 1.1 μg/ml (F) or 10 μg/ml (I). Poly-
d
-lysine samples were tested in a separate experiment from wild-type and mutant III13 samples. Bar, 10 μm.
Figure 13
Stress fiber induction depends on a balance of F7–11 and cationic substrates. Cells were plated on coverslips coated with mixtures of 18 μg/ml F7–11 with varying concentrations of F13 (▵) or on 4.5 μg/ml F7–11 with varying concentrations of F13 (▪), F13(R7S/K25S) (●), or poly-
d
-lysine (○), and stress fiber formation after 2 h was scored.
Similar articles
- A synthetic peptide from the heparin-binding domain III (repeats III4-5) of fibronectin promotes stress-fibre and focal-adhesion formation in melanoma cells.
Moyano JV, Maqueda A, Albar JP, Garcia-Pardo A. Moyano JV, et al. Biochem J. 2003 Apr 15;371(Pt 2):565-71. doi: 10.1042/BJ20021344. Biochem J. 2003. PMID: 12519080 Free PMC article. - Binding of heparin by type III domains and peptides from the carboxy terminal hep-2 region of fibronectin.
Ingham KC, Brew SA, Migliorini MM, Busby TF. Ingham KC, et al. Biochemistry. 1993 Nov 23;32(46):12548-53. doi: 10.1021/bi00097a035. Biochemistry. 1993. PMID: 8241146 - Nucleation of fibronectin fibril assembly requires binding between heparin and the 13th type III module of fibronectin.
Lovett BM, Hill KE, Randolph EM, Wang L, Schwarzbauer JE. Lovett BM, et al. J Biol Chem. 2023 May;299(5):104622. doi: 10.1016/j.jbc.2023.104622. Epub 2023 Mar 17. J Biol Chem. 2023. PMID: 36933809 Free PMC article. - Integrins in cell adhesion and signaling.
Akiyama SK. Akiyama SK. Hum Cell. 1996 Sep;9(3):181-6. Hum Cell. 1996. PMID: 9183647 Review. - Roles of integrins in fibronectin matrix assembly.
Wu C. Wu C. Histol Histopathol. 1997 Jan;12(1):233-40. Histol Histopathol. 1997. PMID: 9046058 Review.
Cited by
- Association between α4 integrin cytoplasmic tail and non-muscle myosin IIA regulates cell migration.
Rivera Rosado LA, Horn TA, McGrath SC, Cotter RJ, Yang JT. Rivera Rosado LA, et al. J Cell Sci. 2011 Feb 1;124(Pt 3):483-92. doi: 10.1242/jcs.074211. Epub 2011 Jan 11. J Cell Sci. 2011. PMID: 21224395 Free PMC article. - An integrative meta-analysis of microRNAs in hepatocellular carcinoma.
ElHefnawi M, Soliman B, Abu-Shahba N, Amer M. ElHefnawi M, et al. Genomics Proteomics Bioinformatics. 2013 Dec;11(6):354-67. doi: 10.1016/j.gpb.2013.05.007. Epub 2013 Nov 25. Genomics Proteomics Bioinformatics. 2013. PMID: 24287119 Free PMC article. - Chlorination and oxidation of human plasma fibronectin by myeloperoxidase-derived oxidants, and its consequences for smooth muscle cell function.
Nybo T, Cai H, Chuang CY, Gamon LF, Rogowska-Wrzesinska A, Davies MJ. Nybo T, et al. Redox Biol. 2018 Oct;19:388-400. doi: 10.1016/j.redox.2018.09.005. Epub 2018 Sep 5. Redox Biol. 2018. PMID: 30237127 Free PMC article. - Titanium alloy surface oxide modulates the conformation of adsorbed fibronectin to enhance its binding to α(5) β(1) integrins in osteoblasts.
Rapuano BE, Lee JJ, MacDonald DE. Rapuano BE, et al. Eur J Oral Sci. 2012 Jun;120(3):185-94. doi: 10.1111/j.1600-0722.2012.954.x. Epub 2012 May 8. Eur J Oral Sci. 2012. PMID: 22607334 Free PMC article. - Tenascin-C suppresses Rho activation.
Wenk MB, Midwood KS, Schwarzbauer JE. Wenk MB, et al. J Cell Biol. 2000 Aug 21;150(4):913-20. doi: 10.1083/jcb.150.4.913. J Cell Biol. 2000. PMID: 10953015 Free PMC article.
References
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. New York: Wiley; 1991.
- Barkalow FJ, Schwarzbauer JE. Localization of the major heparin-binding site in fibronectin. J Biol Chem. 1991;266:7812–7818. - PubMed
- Busby TF, Argraves WS, Brew SA, Pechik I, Gilliland GL, Ingham KC. Heparin binding by fibronectin module III-13 involves six discontinuous basic residues brought together to form a cationic cradle. J Biol Chem. 1995;270:18558–18562. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous