A recombinant measles virus expressing hepatitis B virus surface antigen induces humoral immune responses in genetically modified mice - PubMed (original) (raw)
A recombinant measles virus expressing hepatitis B virus surface antigen induces humoral immune responses in genetically modified mice
M Singh et al. J Virol. 1999 Jun.
Abstract
It has been shown previously that measles virus (MV) can be successfully used to express foreign proteins (M. Singh and M. A. Billeter, J. Gen. Virol. 80:101-106, 1998). To develop an inexpensive MV-based vaccine, we generated recombinant MVs that produce structural proteins of hepatitis B virus (HBV). A recombinant virus that expressed the HBV small surface antigen (HBsAg) was analyzed in terms of its replication characteristics, its genetic stability in cell culture, and its immunogenic potential in genetically modified mice. Although this virus showed a progression of replication slightly slower than that of the parental MV, it appeared to stably maintain the added genetic information; it uniformly expressed the appropriately glycosylated HBsAg after 10 serial passages. Genetically modified mice inoculated with this recombinant MV produced humoral immune responses against both HBsAg and MV proteins.
Figures
FIG. 1
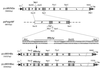
Cloning HBsAg and HBcAg ORFs in p(+)MVNSe antigenomic MV plasmid. ORFs of MV and HBV are shown as rectangles (not to scale) labeled with letters as follows: N, nucleocapsid; P, phosphoprotein; M, matrix; F, fusion; H, hemagglutinin; and L, large protein of MV. Stippled rectangles denote nontranslated regions, and vertical bars denote the nontranscribed intergenic trinucleotides. The triangle labeled “aigr” represents the artificial intergenic region, which consists of gene termination, intergenic, and gene start sequences followed by unique cloning sites. The flanking sequences together with start and stop codons (underlined) of the HBsAg ORF, plasmid names together with total sizes in base pairs, MV antigenomic nucleotide numbers (based on EMBL accession no. Z66517), and restriction sites are as indicated. T7 indicates the T7 RNA polymerase promoter, δ indicates the hepatitis delta virus ribozyme, and TΦ indicates the T7 RNA polymerase terminator.
FIG. 2
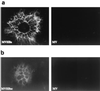
Expression of HBsAg and HBcAg from recombinant MVs. Vero cells were infected with either MVHBs, MVHBsc, or MV-tag-Edm (indicated as MV) and processed for indirect immunofluorescence as described in Materials and Methods. HBVsAg-specific (a) and HBVcAg-specific (b) signals were detected with goat anti-HBsAg and rabbit anti-HBcAg antibodies, respectively, followed by anti-goat- and anti-rabbit antibody–FITC conjugate, respectively.
FIG. 3
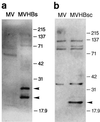
Western immunoblots of HBsAg and HBcAg expressed from recombinant MVs. Vero cells were infected with either MVHBs, MVHBsc, or MV-tag-Edm (indicated as MV), and total cellular proteins were harvested at 24 hpi and processed for Western blots as described in Materials and Methods. The nylon membranes with transferred proteins were developed with goat anti-HBsAg antibodies followed by anti-goat antibody–HRPO conjugate (a) and with rabbit anti-HBcAg antibodies followed by anti-rabbit antibody–HRPO conjugate (b), and proteins were visualized by enhanced chemiluminescence. Two protein bands of approximately 27 and 24 kDa observed in MVHBs cell lysates and a single band of approximately 21 kDa observed in MVHBsc cell lysates are marked with arrowheads. The molecular mass markers (in kilodaltons) are indicated.
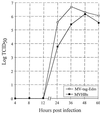
FIG. 4
Growth curve comparison of MVHBs and MV-tag-Edm. Vero cells were infected with MVHBs or MV-tag-Edm at an MOI of 0.01, and the virus was harvested at the indicated time points. The virus titers, expressed as TCID50s per milliliter, are taken from one of three independent experiments; the variations (which were minor) are not shown.
Similar articles
- A vectored measles virus induces hepatitis B surface antigen antibodies while protecting macaques against measles virus challenge.
del Valle JR, Devaux P, Hodge G, Wegner NJ, McChesney MB, Cattaneo R. del Valle JR, et al. J Virol. 2007 Oct;81(19):10597-605. doi: 10.1128/JVI.00923-07. Epub 2007 Jul 18. J Virol. 2007. PMID: 17634218 Free PMC article. - Protective anti-hepatitis B virus responses in rhesus monkeys primed with a vectored measles virus and boosted with a single dose of hepatitis B surface antigen.
Reyes-del Valle J, Hodge G, McChesney MB, Cattaneo R. Reyes-del Valle J, et al. J Virol. 2009 Sep;83(17):9013-7. doi: 10.1128/JVI.00906-09. Epub 2009 Jun 17. J Virol. 2009. PMID: 19535451 Free PMC article. - Hepatitis B virus core-preS2 particles expressed by recombinant vaccinia virus.
Nemecková S, Sroller V, Kunke D, Krystofová J, Kutinová L. Nemecková S, et al. Acta Virol. 1996 Nov-Dec;40(5-6):273-9. Acta Virol. 1996. PMID: 9171455 - DNA-mediated immunization to the hepatitis B surface antigen. Activation and entrainment of the immune response.
Whalen RG, Leclerc C, Dériaud E, Schirmbeck R, Reimann J, Davis HL. Whalen RG, et al. Ann N Y Acad Sci. 1995 Nov 27;772:64-76. doi: 10.1111/j.1749-6632.1995.tb44732.x. Ann N Y Acad Sci. 1995. PMID: 8546414 Review. - Immunogenicity of hybrid hepatitis B surface antigen particles.
Mancini M, Schlienger K, Tiollais P, Michel ML. Mancini M, et al. Int Rev Immunol. 1994;11(2):143-51. doi: 10.3109/08830189409061722. Int Rev Immunol. 1994. PMID: 8046275 Review.
Cited by
- Single-chain antibody displayed on a recombinant measles virus confers entry through the tumor-associated carcinoembryonic antigen.
Hammond AL, Plemper RK, Zhang J, Schneider U, Russell SJ, Cattaneo R. Hammond AL, et al. J Virol. 2001 Mar;75(5):2087-96. doi: 10.1128/JVI.75.5.2087-2096.2001. J Virol. 2001. PMID: 11160713 Free PMC article. - Sequence and immunogenicity of a clinically approved novel measles virus vaccine vector.
Zuniga A, Liniger M, Morin TN, Marty RR, Wiegand M, Ilter O, Weibel S, Billeter MA, Knuchel MC, Naim HY. Zuniga A, et al. Hum Vaccin Immunother. 2013 Mar;9(3):607-13. doi: 10.4161/hv.23242. Epub 2013 Jan 16. Hum Vaccin Immunother. 2013. PMID: 23324616 Free PMC article. - Immunogenicity of a recombinant measles-HIV-1 clade B candidate vaccine.
Stebbings R, Février M, Li B, Lorin C, Koutsoukos M, Mee E, Rose N, Hall J, Page M, Almond N, Voss G, Tangy F. Stebbings R, et al. PLoS One. 2012;7(11):e50397. doi: 10.1371/journal.pone.0050397. Epub 2012 Nov 30. PLoS One. 2012. PMID: 23226275 Free PMC article. - Pediatric measles vaccine expressing a dengue antigen induces durable serotype-specific neutralizing antibodies to dengue virus.
Brandler S, Lucas-Hourani M, Moris A, Frenkiel MP, Combredet C, Février M, Bedouelle H, Schwartz O, Desprès P, Tangy F. Brandler S, et al. PLoS Negl Trop Dis. 2007 Dec 12;1(3):e96. doi: 10.1371/journal.pntd.0000096. PLoS Negl Trop Dis. 2007. PMID: 18160988 Free PMC article. - Immunogenic Subviral Particles Displaying Domain III of Dengue 2 Envelope Protein Vectored by Measles Virus.
Harahap-Carrillo IS, Ceballos-Olvera I, Valle JR. Harahap-Carrillo IS, et al. Vaccines (Basel). 2015 Jul 3;3(3):503-18. doi: 10.3390/vaccines3030503. Vaccines (Basel). 2015. PMID: 26350592 Free PMC article.
References
- Albrecht P, Herrmann K, Burns G R. Role of virus strain in conventional and enhanced measles plaque neutralization test. J Virol Methods. 1981;3:251–260. - PubMed
- Chen R T, Markowitz L E, Albrecht P, Stewart J A, Mofenson L M, Preblud S R, Orenstein W A. Measles antibody: reevaluation of protective titers. J Infect Dis. 1990;162:1036–1042. - PubMed
- Cornu T. Use of measles virus as a vector: stable expression of a supplementary large transcription unit or additional foreign viral glycoproteins. M.S. thesis. Zurich, Switzerland: University of Zurich; 1997.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources