Developmental switch in the short-term modification of unitary EPSPs evoked in layer 2/3 and layer 5 pyramidal neurons of rat neocortex - PubMed (original) (raw)
Developmental switch in the short-term modification of unitary EPSPs evoked in layer 2/3 and layer 5 pyramidal neurons of rat neocortex
A Reyes et al. J Neurosci. 1999.
Abstract
Amplitudes of EPSPs evoked by repetitive presynaptic action potentials can either decrease (synaptic depression) or increase (synaptic facilitation). To determine whether facilitation and depression in the connections between neocortical pyramidal cells varied with the identity of the pre- or the postsynaptic cell and whether they changed during postnatal development, whole-cell voltage recordings were made simultaneously from two or three pyramidal cells in layers 2/3 and 5 of the rat sensorimotor cortex. Unitary EPSPs were evoked when pre- and postsynaptic neurons were in the same and in different layers. In young [postnatal day 14 (P14)] cortex, EPSPs evoked in all connected neurons depressed. The degree of depression was layer specific and was determined by the identity of the presynaptic cell. EPSPs evoked by stimulation of presynaptic layer 5 neurons depressed significantly more than did those evoked by stimulation of layer 2/3 neurons. In mature cortex (P28), however, the EPSPs evoked in these connected neurons facilitated to a comparable degree regardless of the layer in which pre- and postsynaptic neurons were located. The results suggest that in young cortex the degree of synaptic depression in connected pyramidal cells is determined primarily by whether the presynaptic cell was in layer 2/3 or 5 and that maturation of the cortex involves a developmental switch from depression to facilitation between P14 and P28 that eliminates the layer-specific differences. A functional consequence of this switch is that in mature cortex the spread of excitation between neocortical pyramidal neurons is enhanced when action potentials occur in bursts.
Figures
Fig. 1.
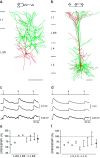
Unitary EPSPs evoked in connected layer 2/3 and in connected layer 5 neocortical pyramidal neurons.a, Camera lucida reconstructions of pyramidal neurons in layer 2/3 from a P14 rat. Three cells (green) innervated a fourth pyramidal cell (red), as indicated in the schematic drawing (inset). Neurons were filled with biocytin during the experiment. Only somata and dendritic arbors were reconstructed. Calibration bar, 100 μm. b, Camera lucida reconstructions of three layer 5 pyramidal neurons from a P14 rat. Two neurons (green) innervated the same postsynaptic neuron (red). In addition, one of the neurons simultaneously innervated two neurons [schematic drawing (inset)]. Calibration bar, 100 μm. c, Unitary EPSPs recorded in the same layer 2/3 pyramidal cell during 10 Hz stimulation of three different presynaptic pyramidal cells.Numbered tic marks in the upper trace_give the times of occurrences of the presynaptic action potentials. The_three lower traces represent averages of EPSPs compiled from 50 to 100 sweeps. The resting membrane potential of the postsynaptic cell was −69 mV. Recordings were obtained from the neurons shown in a. d, Unitary EPSPs recorded in two layer 5 neurons during 10 Hz stimulation of the presynaptic neurons. The circuit is depicted in the_inset_ of b. The timing of presynaptic action potentials is shown in the upper trace. The_second_ and fourth traces from the_top_ show EPSPs evoked in a common target neuron during stimulation of two presynaptic neurons. The second and_third traces_ show EPSPs evoked in two different pyramidal neurons during stimulation of a single presynaptic pyramidal neuron. Resting membrane potentials of the two postsynaptic cells were −62 and −64 mV. e, Summary of the short-term modification of EPSPs evoked in layer 2/3 pyramidal neurons expressed as the ratio of the amplitude of the second EPSP to that of the first EPSP (EPSP2/EPSP1 × 100). Connected squares_represent the amplitude ratios for EPSPs evoked in a common neuron during 10 Hz stimulation of two or more presynaptic pyramidal neurons. The mean (± SD) amplitude ratio of EPSPs (shown on the_right) was 89 ± 17% (n = 16).f, Summary of the amplitude ratios of EPSPs evoked in the same layer 5 neuron during 10 Hz stimulation of two presynaptic layer 5 neurons. The mean (± SD) amplitude ratio (shown on the_right_) was 69 ± 19% (n = 16).
Fig. 2.
EPSPs evoked in layer 5 pyramidal neurons by stimulation of layer 2/3 pyramidal neurons.a, Camera lucida reconstructions of three pyramidal neurons from a P14 rat in which two layer 2/3 pyramidal neurons innervated a common layer 5 pyramidal neuron, as indicated in the schematic drawing (inset). b, Camera lucida reconstructions of three pyramidal neurons from a P14 rat in which a layer 2/3 neuron innervated two layer 5 neurons, as indicated in the schematic drawing (inset). c, Unitary EPSPs evoked in the same layer 5 neuron when two presynaptic neurons located in layer 2/3 were stimulated sequentially at 10 Hz. The stimulation pattern is indicated above each_voltage record_. The resting membrane potential of the postsynaptic neuron was −61 mV. d, Unitary EPSPs evoked simultaneously in two layer 5 neurons when a single presynaptic layer 2/3 neuron was stimulated at 10 Hz. Resting potentials of the postsynaptic cells were −58 and −59 mV. e, Summary of the amplitude ratios (EPSP2/EPSP1 × 100) of EPSPs recorded in layer 5 target neurons. Connected circles represent the amplitude ratios of EPSPs evoked by stimulation of two or more layer 2/3 neurons. The mean (± SD) amplitude ratio (shown on the_right_) is 94 ± 29% (n = 23).f, Summary of the amplitude ratios of EPSPs evoked simultaneously in two layer 5 neurons during stimulation of a single presynaptic layer 2/3 neuron. The mean (± SD) amplitude ratio (shown on the right) is 96 ± 26% (_n_= 8).
Fig. 3.
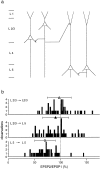
Summary of short-term modification of unitary EPSPs evoked in layer 2/3 and layer 5 pyramidal neurons of young rats.a, Schematic drawing of pyramidal cell connectivity patterns examined by simultaneous double and triple whole-cell recordings from pyramidal neurons located in layers 2/3 and 5.b, Distributions of EPSP amplitude ratios for unitary EPSPs evoked in connections between layer 2/3 neurons (top), between presynaptic layer 2/3 and postsynaptic layer 5 neurons (middle), and between neurons located in layer 5 (bottom) of P14 rats. Symbols above histograms give the mean (± SD) amplitude ratios for each type of connection. The respective values were 97 ± 23% (n = 44; open triangle), 90 ± 25% (n = 55; filled triangle), and 70 ± 20% (n = 52; open circle). Amplitude ratios for EPSPs evoked between layer 2/3 neurons were not significantly different (_p_> 0.1, t test) from those evoked between layer 2/3 and layer 5 neurons. Both were significantly (p< 0.001) greater than those for EPSPs evoked between layer 5 neurons.
Fig. 4.
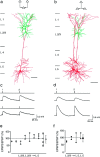
EPSPs evoked simultaneously in layer 2/3 and layer 5 pyramidal neurons of young (P14) rats. a, A layer 2/3 pyramidal cell innervating both a layer 5 pyramidal cell and another layer 2/3 pyramidal cell, as indicated in the schematic drawing (inset). Stimulation of the presynaptic neuron at 10 Hz simultaneously evoked EPSPs in the layer 2/3 neuron (upper trace) and in the layer 5 neuron (lower trace). The stimulation pattern is shown above the_voltage traces_. Resting membrane potentials were −61 mV for the postsynaptic layer 2/3 neuron and −62 mV for the postsynaptic layer 5 neuron. b, Summary of amplitude ratios of EPSPs evoked simultaneously in layer 2/3 (open squares) and layer 5 (filled circles) neurons during stimulation of the same presynaptic layer 2/3 neuron. Mean (± SD) amplitude ratios (shown on the right) for EPSPs evoked in layer 2/3 and layer 5 neurons were 96 ± 37% (open square) and 90 ± 20% (filled circle), respectively. c, A layer 2/3 pyramidal neuron and a layer 5 neuron innervating the same layer 5 neuron, as indicated in the schematic drawing (inset). Stimulation of either the presynaptic layer 2/3 neuron (upper trace) or the layer 5 neuron (lower trace) at 10 Hz evoked EPSPs in the layer 5 target neuron. d, Summary of amplitude ratios for EPSPs recorded in layer 5 neurons during sequential stimulation of layer 2/3 (filled circles) and layer 5 neurons (open circles). Mean (± SD) amplitude ratios (shown on the right) were 93 ± 15% (n = 9; filled circle) and 73 ± 16% (n = 9;open circle) for EPSPs evoked during stimulation of presynaptic layer 2/3 and layer 5 neurons, respectively.
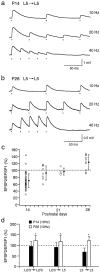
Fig. 5.
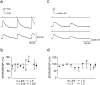
Frequency-dependent short-term modification of EPSPs at different stages of postnatal development. a, In young (P14) cortex, stimulation of a presynaptic layer 5 pyramidal cell at 10, 20, and 40 Hz evoked EPSPs in a postsynaptic layer 5 cell that depressed at all stimulus frequencies. The resting membrane potential was −68 mV. The dots below the voltage traces mark the times of occurrences of presynaptic action potentials. b, In more mature (P28) cortex, stimulation of a presynaptic layer 5 pyramidal neuron evoked EPSPs in a postsynaptic layer 5 cell that facilitated at stimulus frequencies of 10, 20, and 40 Hz. c, Changes in short-term modification of EPSPs evoked in layer 5 neurons in P14 (n = 52), P18 (n = 9), P22 (n = 6), and P28 (n = 10) rats are shown. The filled circles are means (± SD). d, Comparison of means (± SD) of EPSP amplitude ratios for different pyramidal cell connections in layers 2/3 and 5 in P14–P15 (filled bars) and P28 (open bars) animals is shown. EPSP amplitude ratios were measured during 10 Hz stimulation of presynaptic cells. Significant (p < 0.05, two-tailed t_test) differences in the means of the amplitude ratios are marked with_asterisks. The number of paired recordings for P14 and P28 animals was, respectively, 44 and 8 for L2/3 to L2/3 connections, 55 and 7 for L2/3 to L5 connections, and 52 and 9 for L5 to L5 connections.
Similar articles
- Physiology and anatomy of synaptic connections between thick tufted pyramidal neurones in the developing rat neocortex.
Markram H, Lübke J, Frotscher M, Roth A, Sakmann B. Markram H, et al. J Physiol. 1997 Apr 15;500 ( Pt 2)(Pt 2):409-40. doi: 10.1113/jphysiol.1997.sp022031. J Physiol. 1997. PMID: 9147328 Free PMC article. - Target-cell-specific facilitation and depression in neocortical circuits.
Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P, Sakmann B. Reyes A, et al. Nat Neurosci. 1998 Aug;1(4):279-85. doi: 10.1038/1092. Nat Neurosci. 1998. PMID: 10195160 - Excitatory and inhibitory connections show selectivity in the neocortex.
Watts J, Thomson AM. Watts J, et al. J Physiol. 2005 Jan 1;562(Pt 1):89-97. doi: 10.1113/jphysiol.2004.076984. Epub 2004 Nov 11. J Physiol. 2005. PMID: 15539397 Free PMC article. Review. - Deriving excitatory neurons of the neocortex from pluripotent stem cells.
Hansen DV, Rubenstein JL, Kriegstein AR. Hansen DV, et al. Neuron. 2011 May 26;70(4):645-60. doi: 10.1016/j.neuron.2011.05.006. Neuron. 2011. PMID: 21609822 Free PMC article. Review.
Cited by
- Postnatal maturation of GABAergic transmission in the rat basolateral amygdala.
Ehrlich DE, Ryan SJ, Hazra R, Guo JD, Rainnie DG. Ehrlich DE, et al. J Neurophysiol. 2013 Aug;110(4):926-41. doi: 10.1152/jn.01105.2012. Epub 2013 May 29. J Neurophysiol. 2013. PMID: 23719209 Free PMC article. - Surround suppression and sparse coding in visual and barrel cortices.
Sachdev RN, Krause MR, Mazer JA. Sachdev RN, et al. Front Neural Circuits. 2012 Jul 5;6:43. doi: 10.3389/fncir.2012.00043. eCollection 2012. Front Neural Circuits. 2012. PMID: 22783169 Free PMC article. - Timing in the absence of clocks: encoding time in neural network states.
Karmarkar UR, Buonomano DV. Karmarkar UR, et al. Neuron. 2007 Feb 1;53(3):427-38. doi: 10.1016/j.neuron.2007.01.006. Neuron. 2007. PMID: 17270738 Free PMC article. - Learning rules for spike timing-dependent plasticity depend on dendritic synapse location.
Letzkus JJ, Kampa BM, Stuart GJ. Letzkus JJ, et al. J Neurosci. 2006 Oct 11;26(41):10420-9. doi: 10.1523/JNEUROSCI.2650-06.2006. J Neurosci. 2006. PMID: 17035526 Free PMC article. - Quantal release of ATP in mouse cortex.
Pankratov Y, Lalo U, Verkhratsky A, North RA. Pankratov Y, et al. J Gen Physiol. 2007 Mar;129(3):257-65. doi: 10.1085/jgp.200609693. J Gen Physiol. 2007. PMID: 17325196 Free PMC article.
References
- Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science. 1997;275:220–224. - PubMed
- Angevine JB, Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961;192:766–768. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials