Change in the shape of dendritic spines caused by overexpression of drebrin in cultured cortical neurons - PubMed (original) (raw)
Change in the shape of dendritic spines caused by overexpression of drebrin in cultured cortical neurons
K Hayashi et al. J Neurosci. 1999.
Abstract
Dendritic spines are known to be extremely motile, providing a structural mechanism for synaptic plasticity. Actin filaments are thought to be responsible for the changes in the shape of spines. We tested our hypothesis that drebrin, an actin-binding protein, is a regulator of spine shape. In high-density long-term primary cultures of rat cerebral cortex neurons, drebrin was colocalized with actin filaments at spines. We introduced drebrin tagged with green fluorescent protein (GFP) into these neurons to test the ability of exogenous drebrin to localize at spines and the effect of overexpression of drebrin on spine shape. We observed that exogenous drebrin indeed accumulated in spines. But when the actin-binding domain of drebrin was deleted, the protein was distributed in both spines and dendritic shafts, indicating that accumulation of drebrin in the spines required its actin-binding activity. Statistical analysis of the lengths of spines as determined from confocal laser microscopic images revealed that the spines were significantly longer in GFP-drebrin-expressing neurons than in GFP-expressing neurons. The longer spines labeled with GFP-drebrin were demonstrated to be postsynaptic by double labeling of the presynaptic terminals with antibody against synaptophysin. These results directly indicate that drebrin binds to actin filaments at dendritic spines and alters spine shape.
Figures
Fig. 1.
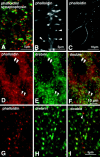
Colocalization of drebrin with actin filaments at spines in primary cultures of cortical neurons at 3 weeks in vitro. A, Confocal observation of neurons that were double-stained with rhodamine–phalloidin and an anti-synaptophysin antibody. Most phalloidin-positive dots are closely associated with synaptophysin-positive dots. B, Confocal observation of a dendrite at an uncrowded area of the culture stained with rhodamine–phalloidin. Phalloidin-positive dots are revealed to be spine heads (arrowheads). C, Confocal observation of the cell body of a neuron stained with rhodamine–phalloidin. D–I, Neurons were double-stained with rhodamine–phalloidin and an anti-drebrin antibody.Yellowish dots in the composite pictures demonstrate the colocalization of drebrin and actin filaments. D–F are observed with an epifluorescent microscope and G–I with a confocal microscope. Single arrowheads in_D–F_ indicate dendritic shafts, and double arrowheads indicate cell soma that was identified using Nomarski optics. The dendritic shafts and cell membranes were weakly stained with rhodamine–phalloidin but hardly with an anti-drebrin antibody. Arrow in I indicates a rare instance in which a phalloidin-positive dot was negative for drebrin.
Fig. 2.
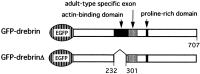
Schematic representation of cDNA constructs. Rat drebrin A with (top) or without (bottom) an actin-binding region was linked to the C terminus of EGFP.Hatched boxes indicate the adult-type-specific exon of drebrin. The proline-rich domain indicated is supposed to be the site of profilin binding. Numbers indicate the amino acid residue numbers of drebrin.
Fig. 3.
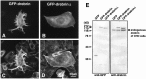
Fluorescence microscopic images and Western blot analysis of CHO cells expressing GFP-fusion proteins. Cells were transfected with GFP-drebrin cDNA (A, C) and GFP-drebrinΔ cDNA (B, D). They were stained with rhodamine–phalloidin to visualize actin filaments and observed using FITC (A, B) and rhodamine (C, D) filter sets. Note that GFP-drebrin bound to actin filaments and caused remodeling of actin filaments, whereas a very small proportion of GFP-drebrinΔ was colocalized with actin filaments, and no remodeling of actin filaments was observed in GFP-drebrinΔ-expressing cells. Western blotting of CHO cells transfected with cDNAs for GFP, GFP-drebrin, or GFP-drebrinΔ is shown in E. Membranes were immunostained with an anti-GFP antibody (left) and an anti-drebrin antibody (right). The position of the endogenous drebrin of CHO cells (embryonic type) is indicated with an arrow.
Fig. 4.
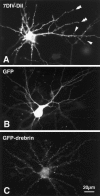
Fluorescence microscopic images of neurons before and 2 weeks after transfection. A, A neuron at 7 d_in vitro_ was labeled with DiI to show morphology before transfection. Arrowheads indicate dendritic filopodia, and double arrowheads indicate dendritic growth cones. Note that principal morphology of dendritic arborization has been already developed. B, A GFP-transfected neuron at 3 weeks in vitro. Fluorescence was seen in cell bodies and dendritic shafts. C, A GFP-drebrin-transfected neuron at 3 weeks in vitro. Fine dots are seen around the dendrites.
Fig. 5.
Confocal microscopic images of neurons expressing GFP-drebrin (A–D), GFP (E), and GFP-drebrinΔ (F). A–D were taken from four different neurons. Fluorescence was concentrated at spines of GFP-drebrin-expressing neurons. In GFP- and GFP-drebrinΔ-expressing neurons, considerable fluorescence was observed in dendritic shafts.
Fig. 6.
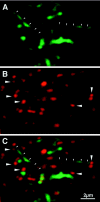
The spines labeled with GFP-drebrin were associated with axonal terminals. A GFP-drebrin-expressing neuron (A) was immunostained with an anti-synaptophysin antibody (B). C shows the composite. The small arrowheads indicate two spines that are ∼5 μm long. The large arrowheads indicate synaptophysin-stained presynaptic terminals that are attached to the spine heads labeled with GFP-drebrin.
Fig. 7.
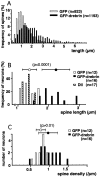
Histograms showing the distribution of length and density of the spines labeled with GFP or GFP-drebrin.A, The length of 50–200 spines was measured in each neuron, giving measurements of 923 in GFP-expressing neurons and 1193 in GFP-drebrin-expressing neurons. B, The average length of spines was calculated for each neuron. The values of 16 GFP-expressing neurons, 12 GFP-drebrin-expressing neurons, and 17 DiI-labeled neurons are represented in a histogram. C, Spine densities of 16 GFP-expressing neurons and 12 GFP-drebrin-expressing neurons are shown. Error bars show SDs.
Similar articles
- Drebrin-dependent actin clustering in dendritic filopodia governs synaptic targeting of postsynaptic density-95 and dendritic spine morphogenesis.
Takahashi H, Sekino Y, Tanaka S, Mizui T, Kishi S, Shirao T. Takahashi H, et al. J Neurosci. 2003 Jul 23;23(16):6586-95. doi: 10.1523/JNEUROSCI.23-16-06586.2003. J Neurosci. 2003. PMID: 12878700 Free PMC article. - Modulatory role of drebrin on the cytoskeleton within dendritic spines in the rat cerebral cortex.
Hayashi K, Ishikawa R, Ye LH, He XL, Takata K, Kohama K, Shirao T. Hayashi K, et al. J Neurosci. 1996 Nov 15;16(22):7161-70. doi: 10.1523/JNEUROSCI.16-22-07161.1996. J Neurosci. 1996. PMID: 8929425 Free PMC article. - The role of drebrin in dendritic spines.
Koganezawa N, Hanamura K, Sekino Y, Shirao T. Koganezawa N, et al. Mol Cell Neurosci. 2017 Oct;84:85-92. doi: 10.1016/j.mcn.2017.01.004. Epub 2017 Feb 1. Mol Cell Neurosci. 2017. PMID: 28161364 Review. - Making of a Synapse: Recurrent Roles of Drebrin A at Excitatory Synapses Throughout Life.
Aoki C, Sherpa AD. Aoki C, et al. Adv Exp Med Biol. 2017;1006:119-139. doi: 10.1007/978-4-431-56550-5_8. Adv Exp Med Biol. 2017. PMID: 28865018 Review.
Cited by
- Differential control of postsynaptic density scaffolds via actin-dependent and -independent mechanisms.
Kuriu T, Inoue A, Bito H, Sobue K, Okabe S. Kuriu T, et al. J Neurosci. 2006 Jul 19;26(29):7693-706. doi: 10.1523/JNEUROSCI.0522-06.2006. J Neurosci. 2006. PMID: 16855097 Free PMC article. - Accelerators, Brakes, and Gears of Actin Dynamics in Dendritic Spines.
Pontrello CG, Ethell IM. Pontrello CG, et al. Open Neurosci J. 2009 Jan 1;3:67-86. doi: 10.2174/1874082000903020067. Open Neurosci J. 2009. PMID: 20463852 Free PMC article. - Activity-dependent redistribution and essential role of cortactin in dendritic spine morphogenesis.
Hering H, Sheng M. Hering H, et al. J Neurosci. 2003 Dec 17;23(37):11759-69. doi: 10.1523/JNEUROSCI.23-37-11759.2003. J Neurosci. 2003. PMID: 14684878 Free PMC article. - Phosphorylation of the actin binding protein Drebrin at S647 is regulated by neuronal activity and PTEN.
Kreis P, Hendricusdottir R, Kay L, Papageorgiou IE, van Diepen M, Mack T, Ryves J, Harwood A, Leslie NR, Kann O, Parsons M, Eickholt BJ. Kreis P, et al. PLoS One. 2013 Aug 5;8(8):e71957. doi: 10.1371/journal.pone.0071957. Print 2013. PLoS One. 2013. PMID: 23940795 Free PMC article. - Interaction of Cupidin/Homer2 with two actin cytoskeletal regulators, Cdc42 small GTPase and Drebrin, in dendritic spines.
Shiraishi-Yamaguchi Y, Sato Y, Sakai R, Mizutani A, Knöpfel T, Mori N, Mikoshiba K, Furuichi T. Shiraishi-Yamaguchi Y, et al. BMC Neurosci. 2009 Mar 24;10:25. doi: 10.1186/1471-2202-10-25. BMC Neurosci. 2009. PMID: 19309525 Free PMC article.
References
- Asada H, Uyemura K, Shirao T. Actin-binding protein, drebrin, accumulates in submembranous regions in parallel with neuronal differentiation. J Neurosci Res. 1994;38:149–159. - PubMed
- Bernstein BW, Bamburg JR. Tropomyosin binding to F-actin protects the F-actin from disassembly by brain actin-depolymerizing factor (ADF). Cell Motil. 1982;2:1–8. - PubMed
- Craven SE, Bredt DS. PDZ proteins organize synaptic signaling pathways. Cell. 1998;93:495–498. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases