Bax, but not Bcl-xL, decreases the lifetime of planar phospholipid bilayer membranes at subnanomolar concentrations - PubMed (original) (raw)
Bax, but not Bcl-xL, decreases the lifetime of planar phospholipid bilayer membranes at subnanomolar concentrations
G Basañez et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Release of proteins through the outer mitochondrial membrane can be a critical step in apoptosis, and the localization of apoptosis-regulating Bcl-2 family members there suggests they control this process. We used planar phospholipid membranes to test the effect of full-length Bax and Bcl-xL synthesized in vitro and native Bax purified from bovine thymocytes. Instead of forming pores with reproducible conductance levels expected for ionic channels, Bax, but not Bcl-xL, created arbitrary and continuously variable changes in membrane permeability and decreased the stability of the membrane, regardless of whether the source of the protein was synthetic or native. This breakdown of the membrane permeability barrier and destabilization of the bilayer was quantified by using membrane lifetime measurements. Bax decreased membrane lifetime in a voltage- and concentration-dependent manner. Bcl-xL did not protect against Bax-induced membrane destabilization, supporting the idea that these two proteins function independently. Corresponding to a physical theory for lipidic pore formation, Bax potently diminished the linear tension of the membrane (i.e., the energy required to form the edge of a new pore). We suggest that Bax acts directly by destabilizing the lipid bilayer structure of the outer mitochondrial membrane, promoting the formation of a pore-the apoptotic pore-large enough to allow mitochondrial proteins such as cytochrome c to be released into the cytosol. Bax could then enter and permeabilize the inner mitochondrial membrane through the same hole.
Figures
Figure 1
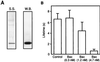
Expression and quantification of Bax and Bcl-xL. (A) Immunoblots showing expression of full-length Bax (Left) and full-length Bcl-xL (Right) by reticulocyte lysate. (Left) Lane 1, filtered reticulocyte lysate with empty vector plasmid added (control); lane 2, filtered reticulocyte lysate after full-length bax plasmid addition; lane 3, truncated Bax, expressed in Escherichia coli, lacking the 22 carboxyl-terminal amino acids. (Right) Lane 1, control; lanes 2 and 3 correspond to two different samples of filtered reticulocyte lysate after Bcl-xL plasmid addition. (B) Quantification of Bax and Bcl-xL expressed by reticulocyte lysate. (Left) A SDS/PAGE gel loaded with known amounts of purified truncated Bax (ΔC22-Bax) and a sample of full-length Bax (F.L. Bax) obtained from the reticulocyte lysate was incubated with a monoclonal antibody (1F6) against the amino terminus of the protein. Full-length Bax was estimated by densitometry using truncated Bax as a standard. (Right) Quantification of full-length Bcl-xL (F.L. Bcl-xL) produced from reticulocyte lysate. Bacterially expressed purified Bcl-xL was used as a standard as above.
Figure 2
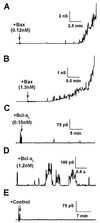
Effect of full-length Bax and Bcl-xL on planar phospholipid bilayer membranes. (A and B) Conductance changes induced by adding filtered reticulocyte lysate to planar phospholipid bilayer membranes formed from a solution of DOPC:DOPE:DOPS (1:1:1) in squalene. Full-length Bax at 0.12 nM (A) and 1.3 nM (B), final concentration. Arrows indicate the time of protein addition. (C and D) Effect of full-length Bcl-xL at two concentrations. Note the different scales. (E) Recording obtained in the presence of the filtered reticulocyte lysate with no Bax or Bcl-xL expressed (control). In all the cases, the holding potential was 40 mV.
Figure 3
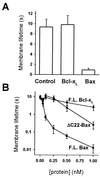
Bax, but not Bcl-xL, decreases membrane lifetime. (A) Lifetimes of DOPC:DOPE:DOPS (1:1:1) bilayers were measured at 250 mV, in the presence of full-length Bax, full-length Bcl-xL, and reticulocyte control. The final concentration of Bax and Bcl-xL was 150 pM. Standard errors are shown for 10–15 experiments. There was no systematic change in lifetime between membranes painted in the presence of Bax and those to which Bax was freshly added. (B) Membrane lifetime as a function of full-length Bax (F.L. Bax), full-length Bcl-xL (F.L. Bcl-xL), and truncated Bax (ΔC22-Bax).
Figure 4
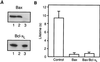
Endogenous Bax from bovine thymus decreases membrane lifetime. (A) Purification of Bax from bovine thymocytes. Bovine Bax was purified from thymocyte soluble protein fraction by conventional column chromatography and α Bax 2D2 immunoaffinity chromatography followed by elution by using a peptide containing the antibody epitope. The resultant eluent was analyzed by SDS/PAGE and silver staining (S.S.) or by Western blotting by using α uBax 2D2 monoclonal antibody (W.B.). (B) Effect of bovine Bax on DOPC:DOPE:DOPS (1:1:1) membrane lifetime. The holding potential was 250 mV.
Figure 5
Bcl-xL does not protect against Bax-induced membrane destabilization. (A) Bax and Bcl-xL do not form stable complexes in the reticulocyte lysate. Bax and Bcl-xL were coexpressed in the reticulocyte, and a sample of the reaction was immunoprecipitated with a monoclonal antibody against Bax. The presence of Bax and Bcl-xL in the reticulocyte lysate (lane 1), immunoprecipitate (lane 2) and in the supernatant (lane 3) was checked by SDS/PAGE and Western blotting, by using monoclonal antibodies against both proteins. (B) Decrease in the lifetime induced by Bax expressed alone or together with Bcl-xL. Bax and Bcl-xL concentrations were 150 pM.
Figure 6
Bax reduces the linear tension of the membrane. (A) Measurement of specific capacitance in the absence and presence of Bax. (B) Effect of Bax on surface tension. Surface tension was found by measuring the change in membrane capacitance that resulted from application of hydrostatic pressure differences between bathing solutions (15). (C) Fitting of the membrane lifetime voltage dependence to Eq. 1, in the presence and absence of Bax (100 pM). The values for A were 0.027 and 0.039 in the absence and presence of Bax, respectively. (D) Linear tension as a function of Bax concentration.
Similar articles
- Investigation of bax-induced release of cytochrome c from yeast mitochondria permeability of mitochondrial membranes, role of VDAC and ATP requirement.
Priault M, Chaudhuri B, Clow A, Camougrand N, Manon S. Priault M, et al. Eur J Biochem. 1999 Mar;260(3):684-91. doi: 10.1046/j.1432-1327.1999.00198.x. Eur J Biochem. 1999. PMID: 10102996 - Bid acts on the permeability transition pore complex to induce apoptosis.
Zamzami N, El Hamel C, Maisse C, Brenner C, Muñoz-Pinedo C, Belzacq AS, Costantini P, Vieira H, Loeffler M, Molle G, Kroemer G. Zamzami N, et al. Oncogene. 2000 Dec 14;19(54):6342-50. doi: 10.1038/sj.onc.1204030. Oncogene. 2000. PMID: 11175349 - Integration and oligomerization of Bax protein in lipid bilayers characterized by single molecule fluorescence study.
Luo L, Yang J, Liu D. Luo L, et al. J Biol Chem. 2014 Nov 14;289(46):31708-31718. doi: 10.1074/jbc.M114.583393. Epub 2014 Oct 6. J Biol Chem. 2014. PMID: 25288797 Free PMC article. - Mitochondrial membrane permeabilisation by Bax/Bak.
Degli Esposti M, Dive C. Degli Esposti M, et al. Biochem Biophys Res Commun. 2003 May 9;304(3):455-61. doi: 10.1016/s0006-291x(03)00617-x. Biochem Biophys Res Commun. 2003. PMID: 12729579 Review. - Regulation of Bax mitochondrial localization by Bcl-2 and Bcl-x(L): keep your friends close but your enemies closer.
Renault TT, Teijido O, Antonsson B, Dejean LM, Manon S. Renault TT, et al. Int J Biochem Cell Biol. 2013 Jan;45(1):64-7. doi: 10.1016/j.biocel.2012.09.022. Epub 2012 Oct 11. Int J Biochem Cell Biol. 2013. PMID: 23064052 Review.
Cited by
- Identification of novel in vivo phosphorylation sites of the human proapoptotic protein BAD: pore-forming activity of BAD is regulated by phosphorylation.
Polzien L, Baljuls A, Rennefahrt UEE, Fischer A, Schmitz W, Zahedi RP, Sickmann A, Metz R, Albert S, Benz R, Hekman M, Rapp UR. Polzien L, et al. J Biol Chem. 2009 Oct 9;284(41):28004-28020. doi: 10.1074/jbc.M109.010702. Epub 2009 Aug 10. J Biol Chem. 2009. PMID: 19667065 Free PMC article. - Peptides derived from apoptotic Bax and Bid reproduce the poration activity of the parent full-length proteins.
García-Sáez AJ, Coraiola M, Dalla Serra M, Mingarro I, Menestrina G, Salgado J. García-Sáez AJ, et al. Biophys J. 2005 Jun;88(6):3976-90. doi: 10.1529/biophysj.104.058008. Epub 2005 Mar 18. Biophys J. 2005. PMID: 15778450 Free PMC article. - Membrane perturbations induced by the apoptotic Bax protein.
Epand RF, Martinou JC, Montessuit S, Epand RM. Epand RF, et al. Biochem J. 2002 Nov 1;367(Pt 3):849-55. doi: 10.1042/BJ20020986. Biochem J. 2002. PMID: 12180909 Free PMC article. - Mitochondria and apoptosis: HQ or high-security prison?
Waterhouse NJ, Green DR. Waterhouse NJ, et al. J Clin Immunol. 1999 Nov;19(6):378-87. doi: 10.1023/a:1020550716138. J Clin Immunol. 1999. PMID: 10634211 Review. - HIV and apoptosis death and the mitochondrion.
Basañez G, Zimmerberg J. Basañez G, et al. J Exp Med. 2001 Feb 19;193(4):F11-4. doi: 10.1084/jem.193.4.f11. J Exp Med. 2001. PMID: 11181706 Free PMC article. No abstract available.
References
- Muchmore S W, Sattler M, Liang H, Meadows R P, Harlan J E, Yoon H S, Nettesheim D, Chang B S, Thompson C B, Wong S L, et al. Nature (London) 1996;381:335–341. - PubMed
- Minn J A, Velez P, Schendel S L, Liang H, Muchmore S W, Fesik S W, Fill M, Thompson C B. Nature (London) 1997;385:353–357. - PubMed
- Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod J J, Mazzei G, et al. Science. 1997;277:370–372. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials