A specific role for group I mGluRs in hippocampal LTP and hippocampus-dependent spatial learning - PubMed (original) (raw)
. 1999 Mar-Apr;6(2):138-52.
Affiliations
- PMID: 10327239
- PMCID: PMC311286
A specific role for group I mGluRs in hippocampal LTP and hippocampus-dependent spatial learning
D Balschun et al. Learn Mem. 1999 Mar-Apr.
Abstract
Metabotropic glutamate receptors (mGluRs) have been implicated in long-term potentiation and in learning and memory formation. In this study, we tested the effects of group I mGluR inhibition on synaptic plasticity and learning of rats at different levels of organization (1) in the hippocampal slice preparation; (2) in freely moving animals implanted with chronic hippocampal electrodes; and (3) in different spatial learning paradigms. To allow a direct comparison of the effects obtained the same doses were used in all paradigms. Bath-application of the selective group I mGluR antagonist (S)4-carboxyphenylglycine (4-CPG) impaired a decremental long-term potentiation (LTP) induced by a weak tetanization paradigm, but failed to affect a robust LTP generated by strong tetanization. In contrast, 4-CPG impaired a robust LTP in freely moving animals if applied 30 min before tetanization. The same dose of 4-CPG only impeded spatial learning mildly in the eight-arm radial maze and had no effect on a simple configuration of the Y-maze spatial alternation task. In the more difficult configuration of this task, however, 4-CPG caused complete amnesia. The lack of state-dependent 4-CPG actions and the absence of any 4-CPG effects in the open-field test classify the obtained retention deficit as a selective impairment of memory storage. Our results indicate a specific role of group I mGluRs in certain types of synaptic plasticity and of spatial learning.
Figures
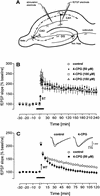
Figure 1
Under in vitro conditions, the mGluR group I antagonist 4-CPG impairs LTP induced by weak tetanization (WT) but not by strong tetanization (ST). (A) Scheme of the placement of recording electrodes in the CA1 region. (B) 4-CPG applied in increasing concentrations does not impair a potentiation induced by a strong tetanization paradigm (ST; 10 bursts of four stimuli at 100 Hz, separated by 200 msec) but was effective (C) if a weak tetanization protocol (WT; 100 Hz, 400-msec duration) was used to generate LTP. Analog traces depict typical responses taken immediately before tetanization (broken line) and 60 min thereafter (solid line). Arrows indicate the time of tetanization and horizontal bars indicate the bath application of 4-CPG.
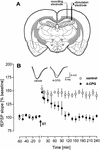
Figure 2
4-CPG Applied ICV to freely moving animals impaired effectively an LTP induced by a strong tetanization paradigm (ST; 10 bursts of 10 pulses, 100 Hz, interburst interval 10 sec, 0.1-msec duration each stimulus). (A) Schematic diagram of electrode placement for the recording from the CA1 area of the right hemisphere. (B) Time course of the potentiation of the fEPSP slope. Analog traces represent typical recordings from a 4-CPG-treated animal (right) and a control animal (left), taken 5 min (solid line) and 240 min (broken line) after tetanization. The arrow indicates the time of tetanization.
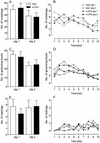
Figure 3
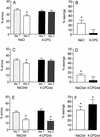
The application of 4-CPG had no influence on behavior in the open field. Bars depict the mean number of crossings (A), grooming bouts (C), and rearings (E), as calculated for each day of testing. In the graphs (B, D, and F, respectively) at the 1-min time courses of the same parameters are given. The only between-group difference that was statistically significant was in the rearing behavior of the 4-CPG group and controls at the seventh minute of day 1 (P < 0.05).
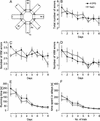
Figure 4
4-CPG only mildly affected spatial learning in the eight-arm RAM. The means of two sessions per day are given. The only difference that was detected concerns the number of reference memory (RM) errors, which was higher on day 1 and day 8 in 4-CPG animals than in controls (C) (P < 0.05). In the schematic of the eight-arm RAM shown in A the dotted lines indicate the triple-beam infrared detector. (BA) Baited arm; (FC) food cup; (IPS) infrared photosensors; (WM) working memory.
Figure 5
The effect of 4-CPG on learning of the SAT was contingent on the difficulty of the particular task configuration. (A–D) Effects of 4-CPG in the more difficult configuration of SAT (dSAT), including a transfer of the experimental animals to another alley after half on the session (20 runs). (A,B) Under these conditions, the ICV application of 4-CPG before the training session prevented the decline of errors on day 2 resulting in percent savings that were not significantly different from zero. Controls, in contrast, displayed a significant retention on the second day. (C,D) The impairment of SAT learning was not state-dependent (sd), as the effects of 4-CPG given before both the training and the retention session (4-CPG/sd) resembled the findings obtained after application of the drug before training depicted above. (E,F) Omission of the transfer (ot) after half of the session (20 runs) resulted in a configuration of SAT that could be acquired more easily (eSAT) as indicated by the higher percent savings as compared with dSAT. This configuration abolished the effect of 4-CPG.
Similar articles
- When are class I metabotropic glutamate receptors necessary for long-term potentiation?
Wilsch VW, Behnisch T, Jäger T, Reymann KG, Balschun D. Wilsch VW, et al. J Neurosci. 1998 Aug 15;18(16):6071-80. doi: 10.1523/JNEUROSCI.18-16-06071.1998. J Neurosci. 1998. PMID: 9698302 Free PMC article. - Antagonism of group III metabotropic glutamate receptors results in impairment of LTD but not LTP in the hippocampal CA1 region, and prevents long-term spatial memory.
Altinbilek B, Manahan-Vaughan D. Altinbilek B, et al. Eur J Neurosci. 2007 Sep;26(5):1166-72. doi: 10.1111/j.1460-9568.2007.05742.x. Eur J Neurosci. 2007. PMID: 17767495 - Comparing the role of metabotropic glutamate receptors in long-term potentiation and in learning and memory.
Riedel G, Wetzel W, Reymann KG. Riedel G, et al. Prog Neuropsychopharmacol Biol Psychiatry. 1996 Jul;20(5):761-89. doi: 10.1016/0278-5846(96)00058-9. Prog Neuropsychopharmacol Biol Psychiatry. 1996. PMID: 8870063 Review. - Metabotropic glutamate receptors in hippocampal long-term potentiation and learning and memory.
Riedel G, Reymann KG. Riedel G, et al. Acta Physiol Scand. 1996 May;157(1):1-19. doi: 10.1046/j.1365-201X.1996.484231000.x. Acta Physiol Scand. 1996. PMID: 8735650 Review.
Cited by
- Coordinated activation of distinct Ca(2+) sources and metabotropic glutamate receptors encodes Hebbian synaptic plasticity.
Tigaret CM, Olivo V, Sadowski JHLP, Ashby MC, Mellor JR. Tigaret CM, et al. Nat Commun. 2016 Jan 13;7:10289. doi: 10.1038/ncomms10289. Nat Commun. 2016. PMID: 26758963 Free PMC article. - Promiscuous involvement of metabotropic glutamate receptors in the storage of _N_-methyl-d-aspartate receptor-dependent short-term potentiation.
Ingram R, Volianskis A. Ingram R, et al. Philos Trans R Soc Lond B Biol Sci. 2024 Jul 29;379(1906):20230445. doi: 10.1098/rstb.2023.0445. Epub 2024 Jun 10. Philos Trans R Soc Lond B Biol Sci. 2024. PMID: 38853548 Free PMC article. - Involvement of the metabotropic glutamate receptor mGluR5 in NMDA receptor-dependent, learning-facilitated long-term depression in CA1 synapses.
Popkirov SG, Manahan-Vaughan D. Popkirov SG, et al. Cereb Cortex. 2011 Mar;21(3):501-9. doi: 10.1093/cercor/bhq093. Epub 2010 Jun 4. Cereb Cortex. 2011. PMID: 20525770 Free PMC article. - Activation of TRPC1 Channel by Metabotropic Glutamate Receptor mGluR5 Modulates Synaptic Plasticity and Spatial Working Memory.
Lepannetier S, Gualdani R, Tempesta S, Schakman O, Seghers F, Kreis A, Yerna X, Slimi A, de Clippele M, Tajeddine N, Voets T, Bon RS, Beech DJ, Tissir F, Gailly P. Lepannetier S, et al. Front Cell Neurosci. 2018 Sep 14;12:318. doi: 10.3389/fncel.2018.00318. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30271326 Free PMC article. - G(alpha)q-deficient mice lack metabotropic glutamate receptor-dependent long-term depression but show normal long-term potentiation in the hippocampal CA1 region.
Kleppisch T, Voigt V, Allmann R, Offermanns S. Kleppisch T, et al. J Neurosci. 2001 Jul 15;21(14):4943-8. doi: 10.1523/JNEUROSCI.21-14-04943.2001. J Neurosci. 2001. PMID: 11438569 Free PMC article.
References
- Aiba A, Chen C, Herrup K, Rosenmund C, Stevens CF, Tonegawa S. Reduced hippocampal long-term potentiation and context-specific deficit in associative learning in mGluR1 mutant mice. Cell. 1994;79:365–375. - PubMed
- Artola A, Singer W. Long-term depression of excitatory synaptic transmission and its relationship to long-term potentiation. Trends Neurosci. 1993;16:480–487. - PubMed
- Bashir ZI, Bortolotto ZA, Davies CH, Beretta N, Irving AJ, Sea AJ, Henley JM, Jane DE, Watkins JC, Collingridge GL. Induction of LTP in the hippocampus needs synaptic activation of glutamate metabotropic receptors. Nature. 1993;363:347–350. - PubMed
- Behnisch T, Reymann KG. Co-activation of metabotropic glutamate and N-methyl-D-aspartate receptors is involved in mechanisms of long-term potentiation maintenance in rat hippocampal CA1 neurons. Neuroscience. 1993;54:37–47. - PubMed
- Ben-Ari Y, Aniksztejn L, Bregestowski P. Protein kinase-C modulation of NMDA currents—an important link for LTP induction. Trends Neurosci. 1992;15:333–339. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources