VEGFR-3 and its ligand VEGF-C are associated with angiogenesis in breast cancer - PubMed (original) (raw)
VEGFR-3 and its ligand VEGF-C are associated with angiogenesis in breast cancer
R Valtola et al. Am J Pathol. 1999 May.
Abstract
Recently, monoclonal antibodies against the human vascular endothelial growth factor receptor VEGFR-3 were shown to provide a specific antigenic marker for lymphatic endothelium in various normal tissues. In this study we have investigated the expression of VEGFR-3 and its ligand VEGF-C in normal breast tissue and in breast tumors by immunohistochemistry. VEGFR-3 was weakly expressed in capillaries of normal breast tissue and in fibroadenomas. In intraductal breast carcinomas, VEGFR-3 was prominent in the "necklace" vessels adjacent to the basal lamina of the tumor-filled ducts. VEGF receptor 1 and 2 as well as blood vessel endothelial and basal lamina markers were colocalized with VEGFR-3 in many of these vessels. Antibodies against smooth muscle alpha-actin gave a weak staining of the necklace vessels, suggesting that they were incompletely covered by pericytes/smooth muscle cells. A highly elevated number of VEGFR-3 positive vessels was found in invasive breast cancer in comparison with histologically normal breast tissue (P < 0.0001, the Mann-Whitney test). VEGF-C was located in the cytoplasm of intraductal and invasive cancer cells. The results demonstrate that the expression of VEGFR-3 becomes up-regulated in the endothelium of angiogenic blood vessels in breast cancer. The results also suggest that VEGF-C secreted by the intraductal carcinoma cells acts predominantly as an angiogenic growth factor for blood vessels, although this paracrine signaling network between the cancer cells and the endothelium may also be involved in modifying the permeabilities of both blood and lymphatic vessels and metastasis formation.
Figures
Figure 1.
Immunostaining for VEGFR-3 (A), PAL-E (B), and collagen XVIII (C) in adjacent sections of histologically normal breast tissue. Careful examination of the staining patters shows that most of the weakly VEGFR-3 positive vessels are also stained for PAL-E and collagen XVIII (black arrows). One of the vessels, indicated by green arrows, is stained for VEGFR-3 but not for the PAL-E and only very weakly for collagen XVIII and therefore presumably lymphatic. In a benign fibroadenoma, numerous VEGFR-3 positive vessels can be observed (arrows in D). Magnifications, ×400.
Figure 2.
Immunohistochemical characterization of VEGFR-3 expressing vessels in intraductal carcinoma. In adjacent sections (A, B), VEGFR-3 and PAL-E decorate a similar pattern of necklace vessels (arrowheads) around the duct filled with carcinoma cells. In double staining for VEGFR-3 and Ki-67, several nucleae of the tumor cells inside the ducts are strongly positive for Ki-67 (A, inset, green arrows) as are several endothelial cell nucleae of the necklace vessels (red arrow). Some distance away, an apparently quiescent, VEGFR-3 positive vessel with a Ki-67 negative nucleus is marked with a black arrow. Another set of adjacent sections was compared with staining for VEGFR-3 (C), laminin (D), collagen XVIII (E), and SMA (F). Double staining for PAL-E and VEGFR-3 (G) and comparison with adjacent section stained for VEGFR-3 only (H). The vessels adjacent to the affected ducts are double-positive (arrowheads), whereas a VEGFR-3 positive vessel is present a short distance away from the affected duct in the interductal stroma (arrows). Note that basal lamina is positive for PAL-E in the double staining procedure. Magnification, ×400 (A, B); ×320 (C, D, E, F); ×480 (G, H).
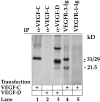
Figure 3.
Analysis of anti-VEGF-C antibodies by immunoprecipitation. Metabolically labeled conditioned medium of 293 T cells transfected with expression vectors for VEGF-C or VEGF-D was precipitated with the indicated antibodies (α-) or receptor bodies (-Ig) detailed in the materials and methods and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography. Note the lack of precipitation of VEGF-D by the anti-VEGF-C antibodies. Note also that the VEGFR-3-Ig precipitates both 29/31 kd and 21.5 kd forms of VEGF-C, which are not recognized by VEGFR-1-Ig.
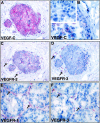
Figure 4.
Comparison of VEGF-C (A, B) and VEGFR (C-F) stainings in intraductal carcinoma. Note the expression of VEGF-C in all (A) or part (B) of the carcinoma in situ cells. Negative control using anti-VEGF-C antibodies blocked with a 10-fold molar excess of the VEGF-C protein gave no staining of the carcinoma cells (inset in B). C and D show sections adjacent to A stained for VEGFR-2 and VEGFR-3, respectively. Note that the necklace vessels are positive for both VEGFR-2 and VEGFR-3 in the vicinity of VEGF-C expressing carcinoma cells. E and F show a similar comparison for VEGFR-1 and VEGFR-3, respectively. Note that the staining for VEGFR-3 appears more discontinuous. Magnification, ×300 (<label;A, C, D>); ×400 (B); ×350 (E, F).
Figure 5.
VEGFR-3 in invasive ductal (A-C) and lobular (D) breast cancer. The intratumoral VEGFR-3 positive vessels (A) surround tumor cell clusters in ductal breast carcinoma and are predominantly positive for PAL-E (black arrows in B). Also an invasive growth pattern is associated with VEGFR-3 positive vessels (C), whereas no staining is observed using anti-VEGFR-3 antibodies blocked with a 10-fold molar excess of the VEGFR-3 protein (inset in C). Occasionally, invasion of the carcinoma cells into the VEGFR-3 positive vessels could be observed (D). Magnification, ×200.
Similar articles
- The short form of the alternatively spliced flt-4 but not its ligand vascular endothelial growth factor C is related to lymph node metastasis in human breast cancers.
Gunningham SP, Currie MJ, Han C, Robinson BA, Scott PA, Harris AL, Fox SB. Gunningham SP, et al. Clin Cancer Res. 2000 Nov;6(11):4278-86. Clin Cancer Res. 2000. PMID: 11106244 - Vascular endothelial growth factors C and D and their VEGFR-2 and 3 receptors in blood and lymphatic vessels in healthy and arthritic synovium.
Paavonen K, Mandelin J, Partanen T, Jussila L, Li TF, Ristimaki A, Alitalo K, Konttinen YT. Paavonen K, et al. J Rheumatol. 2002 Jan;29(1):39-45. J Rheumatol. 2002. PMID: 11824969 - Vascular stroma formation in carcinoma in situ, invasive carcinoma, and metastatic carcinoma of the breast.
Brown LF, Guidi AJ, Schnitt SJ, Van De Water L, Iruela-Arispe ML, Yeo TK, Tognazzi K, Dvorak HF. Brown LF, et al. Clin Cancer Res. 1999 May;5(5):1041-56. Clin Cancer Res. 1999. PMID: 10353737 - Lymphatic versus blood vascular endothelial growth factors and receptors in humans.
Partanen TA, Paavonen K. Partanen TA, et al. Microsc Res Tech. 2001 Oct 15;55(2):108-21. doi: 10.1002/jemt.1162. Microsc Res Tech. 2001. PMID: 11596156 Review. - Lymphangiogenesis and breast cancer metastasis.
Cunnick GH, Jiang WG, Gomez KF, Mansel RE. Cunnick GH, et al. Histol Histopathol. 2002;17(3):863-70. doi: 10.14670/HH-17.863. Histol Histopathol. 2002. PMID: 12168797 Review.
Cited by
- Vascular endothelial growth factor-D is an independent prognostic factor in epithelial ovarian carcinoma.
Yokoyama Y, Charnock-Jones DS, Licence D, Yanaihara A, Hastings JM, Holland CM, Emoto M, Umemoto M, Sakamoto T, Sato S, Mizunuma H, Smith SK. Yokoyama Y, et al. Br J Cancer. 2003 Jan 27;88(2):237-44. doi: 10.1038/sj.bjc.6600701. Br J Cancer. 2003. PMID: 12610509 Free PMC article. - Small molecule chloropyramine hydrochloride (C4) targets the binding site of focal adhesion kinase and vascular endothelial growth factor receptor 3 and suppresses breast cancer growth in vivo.
Kurenova EV, Hunt DL, He D, Magis AT, Ostrov DA, Cance WG. Kurenova EV, et al. J Med Chem. 2009 Aug 13;52(15):4716-24. doi: 10.1021/jm900159g. J Med Chem. 2009. PMID: 19610651 Free PMC article. - Soluble Vegfr3 gene therapy suppresses multi-organ metastasis in a mouse mammary cancer model.
Shibata MA, Shibata E, Tanaka Y, Shiraoka C, Kondo Y. Shibata MA, et al. Cancer Sci. 2020 Aug;111(8):2837-2849. doi: 10.1111/cas.14531. Epub 2020 Jul 4. Cancer Sci. 2020. PMID: 32539229 Free PMC article. - Dysregulation of Lymphatic Endothelial VEGFR3 Signaling in Disease.
Kuonqui K, Campbell AC, Sarker A, Roberts A, Pollack BL, Park HJ, Shin J, Brown S, Mehrara BJ, Kataru RP. Kuonqui K, et al. Cells. 2023 Dec 28;13(1):68. doi: 10.3390/cells13010068. Cells. 2023. PMID: 38201272 Free PMC article. Review. - Mammary cancer gene therapy targeting lymphangiogenesis: VEGF-C siRNA and soluble VEGF receptor-2, a splicing variant.
Shibata MA, Ambati J, Shibata E, Yoshidome K, Harada-Shiba M. Shibata MA, et al. Med Mol Morphol. 2012 Dec;45(4):179-84. doi: 10.1007/s00795-012-0576-5. Epub 2012 Dec 7. Med Mol Morphol. 2012. PMID: 23224595 Review.
References
- Ferrara N, Davis-Smyth T: The biology of vascular endothelial growth factor. Endocr Rev 1997, 18:4-25 - PubMed
- Shibuya M: Role of VEGF-FLT receptor system in normal and tumor angiogenesis. Adv Cancer Res 1995, 67:281-316 - PubMed
- Ferrara N: The role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int 1999, In press - PubMed
- Clauss M, Weich H, Breier G, Knies U, Rockl W, Waltenberger J, Risau W: The vascular endothelial growth factor receptor Flt-1 mediates biological activities: Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J Biol Chem 1996, 271:17629-17634 - PubMed
- Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N: Inhibition of vascular endothelial growth factor induced angiogenesis suppresses tumour growth in vivo. Nature 1993, 362:841-844 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous