Protein kinase Cdelta mediates neurogenic but not mitogenic activation of mitogen-activated protein kinase in neuronal cells - PubMed (original) (raw)
Protein kinase Cdelta mediates neurogenic but not mitogenic activation of mitogen-activated protein kinase in neuronal cells
K C Corbit et al. Mol Cell Biol. 1999 Jun.
Abstract
In several neuronal cell systems, fibroblast-derived growth factor (FGF) and nerve growth factor (NGF) act as neurogenic agents, whereas epidermal growth factor (EGF) acts as a mitogen. The mechanisms responsible for these different cellular fates are unclear. We report here that although FGF, NGF, and EGF all activate mitogen-activated protein (MAP) kinase (extracellular signal-related kinase [ERK]) in rat hippocampal (H19-7) and pheochromocytoma (PC12) cells, the activation of ERK by the neurogenic agents FGF and NGF is dependent upon protein kinase Cdelta (PKCdelta), whereas ERK activation in response to the mitogenic EGF is independent of PKCdelta. Antisense PKCdelta oligonucleotides or the PKCdelta-specific inhibitor rottlerin inhibited FGF- and NGF-induced, but not EGF-induced, ERK activation. In contrast, EGF-induced ERK activation was inhibited by the phosphatidylinositol-3-kinase inhibitor wortmannin, which had no effect upon FGF-induced ERK activation. Rottlerin also inhibited the activation of MAP kinase kinase (MEK) in response to activated Raf, but had no effect upon c-Raf activity or ERK activation by activated MEK. These results indicate that PKCdelta functions either downstream from or in parallel with c-Raf, but upstream of MEK. Inhibition of PKCdelta also blocked neurite outgrowth induced by FGF and NGF in PC12 cells and by activated Raf in H19-7 cells, indicating a role for PKCdelta in the neurogenic effects of FGF, NGF, and Raf. Interestingly, the PKCdelta requirement is apparently cell type specific, since FGF-induced ERK activation was independent of PKCdelta in NIH 3T3 murine fibroblasts, in which FGF is a mitogen. These data demonstrate that PKCdelta contributes to growth factor specificity and response in neuronal cells and may also promote cell-type-specific differences in growth factor signaling.
Figures
FIG. 1
PKCδ is expressed in H19-7 cells and is selectively activated by neurogenic factors in H19-7 and PC12 cells. (A) H19-7 cells in N2 medium at 39°C were either untreated (CTRL), stimulated with 10 ng of FGF per ml for 10 min, pretreated with 400 nM PDBu for 24 h, or pretreated with 5 μM rottlerin (Rott) for 5 h. Cells were lysed, and equal protein aliquots were resolved by SDS-PAGE (10% polyacrylamide) and then immunoblotted with anti-PKCδ antibody. (B) H19-7 cells were either untreated (CTRL), stimulated with 10 ng of EGF per ml, stimulated with 10 ng of FGF per ml, or pretreated with 5 μM rottlerin for 6 h prior to stimulation with 10 ng of FGF per ml for 10 min. The cells were lysed and fractionated into cytosolic (C) and membrane (M) fractions and immunoblotted with anti-PKCδ antibody as described in Materials and Methods.
FIG. 2
Effects of rottlerin and wortmannin on FGF- and EGF-induced ERK activation in H19-7 cells. (A) H19-7 cells in N2 medium at 39°C were pretreated with the indicated dose of rottlerin and then stimulated with 10 ng of FGF per ml for 10 min. After lysis, equal protein aliquots were resolved by SDS-PAGE (10% polyacrylamide) and then immunoblotted with anti-phospho-ERK antibody. (B) Cells were treated as for panel A except that 10 ng of EGF per ml rather than FGF was used for stimulation. (C) H19-7 cells in N2 medium at 39°C were pretreated with the indicated dose of wortmannin for 15 min and then stimulated with 10 ng of FGF per ml for 10 min. After lysis, equal protein aliquots were resolved by SDS-PAGE (10% polyacrylamide) and then immunoblotted with anti-phospho-ERK antibody. (D) Cells were treated for panel C, except that 10 ng of EGF per ml rather than FGF was used for stimulation.
FIG. 3
Effects of various inhibitors on FGF- and EGF-induced ERK activation in H19-7 cells. (A) H19-7 cells in N2 medium at 39°C were either untreated (CTRL) or were pretreated with 400 nM PDBu for 24 h, 1 μM Gö 6976 (Go) for 2 h, 5 μM rottlerin (Rott) for 6 h, 200 nM wortmannin (Wort) for 15 min, 1 μM chelerythrine chloride (CC) for 2 h, or 30 μM MEK inhibitor PD98059 (MI) for 15 min. Cells were then stimulated with 10 ng of FGF per ml for 10 min. After lysis, equal protein aliquots were resolved by SDS-PAGE (10% polyacrylamide) and then immunoblotted with anti-phospho-ERK antibody. (B) Cells were treated as in for panel A, except that they were stimulated with 10 ng of EGF per ml rather than FGF.
FIG. 4
PKCɛ is expressed in H19-7 cells and is constitutively associated with the membrane fraction. (A) Expression of PKC isozymes in H19-7 cells. H19-7 cells were lysed, and equal protein aliquots were resolved by SDS-PAGE (10% polyacrylamide). Samples were immunoblotted with antibodies to PKCα, PKCβ, PKCγ, PKCθ, PKCη, PKCɛ, PKCδ, PKCζ, PKCι, and PKCλ. (B) H19-7 cells were either untreated (CTRL) or were stimulated with 10 ng of FGF per ml or pretreated with 5 μg of rottlerin for 6 h prior to stimulation with 10 ng of FGF per ml for 10 min (Rott + FGF). The cells were lysed and fractionated into cytosolic (C) and membrane (M) fractions and immunoblotted with anti-PKCɛ antibody as described in Materials and Methods.
FIG. 5
Effects of various inhibitors on ERK activation in ΔRaf-1:ER and PC12 cells. (A) ΔRaf-1:ER cells were either untreated (CTRL) or were pretreated with 400 nM PDBu for 24 h, 1 μM Gö 6976 (Go) for 2 h, 5 μM rottlerin (Rott) for 6 h, or 1 μM chelerythrine chloride (CC) for 2 h and then stimulated with (+) or without (−) 1 μM estradiol for 30 min. After cell lysis, equal protein aliquots were resolved by SDS-PAGE (10% polyacrylamide) and immunoblotted with anti-phospho-ERK antibody. (B) PC12 cells were either untreated (CTRL) or were pretreated with 400 nM PDBu (PDBu) for 24 h or 5 μM rottlerin (Rott) for 6 h and then stimulated with 10 ng of EGF, FGF, or NGF per ml for 10 min. Samples were then processed as for panel A.
FIG. 6
Rottlerin does not block FGF- or EGF-induced ERK activation in NIH 3T3 cells. (A) Effect of FGF or EGF on ERK phosphorylation. NIH 3T3 cells were either untreated (CTRL) or were stimulated with 10 ng of EGF or FGF per ml for 10 min in the presence or absence of 5 μM rottlerin (Rott). Cells were lysed, resolved by SDS-PAGE (10% polyacrylamide), and then immunoblotted with anti-phospho-ERK. (B) Expression of PKCδ in NIH 3T3 cells. Cells were treated as for panel A, lysed, resolved by SDS-PAGE (10% polyacrylamide), and then immunoblotted with anti-PKCδ antibody.
FIG. 7
PKCδ but not PKCɛ is required for FGF-induced ERK activation. (A) Antisense (AS) PKCδ phosphorothioate ODNs block PKCδ expression and ERK activation in H19-7 cells. Cells were pretreated with either sense or antisense PKCδ ODNs as described in Materials and Methods and then either left untreated (CTRL) or were stimulated with 10 ng of FGF per ml for 10 min. Cells were then lysed, and the lysates were resolved by SDS-PAGE (10% polyacrylamide) and assayed for PKCδ expression by immunoblotting with anti-PKCδ antibody. MAP kinase activation was assayed by immunoblotting with anti-phospho-ERK antibody. (B) Antisense PKCɛ phosphorothioate oligonucleotides block PKCɛ expression, but do not inhibit ERK activation in H19-7 cells. Cells were pretreated with either sense or antisense PKCɛ oligonucleotides as described in Materials and Methods and then either were left untreated or were stimulated with 10 ng of FGF per ml for 10 min. Cells were then lysed, and the lysates were resolved by SDS-PAGE (10% polyacrylamide) and assayed for PKCɛ expression by immunoblotting with anti-PKCɛ antibody. MAP kinase activation was assayed by immunoblotting with anti-phospho-ERK antibody. (C) Antisense PKCδ phosphorothioate oligonucleotides block PKCδ expression and ERK activation by NGF in PC12 cells. PC12 cells were treated and analyzed as for panel A, except that 50 ng of NGF per ml was used instead of FGF. (D) Antisense PKCɛ phosphorothioate oligonucleotides block PKCɛ expression but do not inhibit ERK activation by NGF in PC12 cells. PC12 cells were treated and analyzed as for panel B, except that 50 ng of NGF per ml was used instead of FGF.
FIG. 8
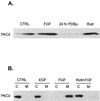
Rottlerin (Rott) does not block FGF-induced c-Raf, FLAG-Raf, or estradiol-induced ΔRaf-1:ER kinase activity. (A) H19-7 cells were transfected with either control vector or an expression vector for FLAG-tagged c-Raf. The cells were then left untreated (CTRL), pretreated with 5 μM rottlerin for 6 h, and/or stimulated with 10 ng of FGF per ml for 10 min. Following treatment, cells were lysed, and FLAG-Raf was immunoprecipitated with anti-FLAG antibody. The samples were resolved by SDS-PAGE (10% polyacrylamide) and assayed for Raf kinase activity by using inactive MEK as a substrate as described in Materials and Methods. The immunoprecipitated Raf was quantitated by immunoblotting with anti-FLAG antibody. DMSO, dimethyl sulfoxide. (B) H19-7 cells were left untreated, pretreated with 5 μM rottlerin for 6 h, and/or stimulated with 10 ng of FGF per ml for 10 min. Following treatment, cells were lysed, and endogenous c-Raf was immunoprecipitated with anti-c-Raf antibody. The samples were resolved by SDS-PAGE (10% polyacrylamide) and assayed for Raf kinase activity by using inactive MEK as a substrate as described in Materials and Methods. The immunoprecipitated Raf was quantitated by immunoblotting with anti-c-Raf antibody. (C) ΔRaf-1:ER cells were either untreated or were stimulated with 1 μM estradiol for 30 min. The cells were then lysed, and the ΔRaf-1:ER was immunoprecipitated with anti-ER antibodies and assayed for Raf kinase activity by using inactive MEK as a substrate as described in Materials and Methods. Immunoprecipitated ΔRaf-1:ER was quantitated by immunoblotting with anti-ER antibody.
FIG. 9
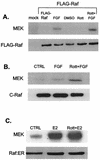
Activation of MEK but not ERK requires PKCδ. (A) PKCδ inhibitors block MEK activation by FGF but not EGF in H19-7 cells. Cells were either untreated (CTRL) or were pretreated with 1 μM Gö 6976 (Go) for 2 h, 5 μM rottlerin (Rott) for 6 h, 1 μM chelerythrine chloride (CC) for 2 h, or 30 μM MEK inhibitor PD98059 (MI) for 15 min. Cells were then stimulated with 10 ng of either FGF or EGF per ml for 10 min. After cell lysis, equal protein aliquots were resolved by SDS-PAGE (10% polyacrylamide). MEK activation was assayed by immunoblotting with anti-phospho-MEK (P-MEK) antibody. (B) PKCδ inhibitors block MEK activation by estradiol in ΔRaf-1:ER cells. Cells were pretreated as for panel A and then exposed to 1 μM estradiol for 30 min. Samples were resolved by SDS and assayed for MEK activation as for panel A. (C) PKCδ inhibitors block MEK activation by FGF but not EGF in PC12 cells. PC12 cells were treated and processed as for panel A. (D) Rottlerin does not block activation of ERK by constitutively activated MEK. H19-7 cells were mock transfected or cotransfected with an expression vector for HA-ERK2 and MEK-2E, a constitutively activated MEK. Cells were then left untreated or were pretreated with 5 μM rottlerin for 6 h or stimulated with 10 ng of FGF per ml as indicated. Following treatment, cells were lysed, and HA-ERK was immunoprecipitated with anti-HA antibody. The immunoprecipitated HA-ERK was assayed for kinase activity by using MBP as a substrate as described in Materials and Methods, and the reaction products were resolved by SDS-PAGE (10% polyacrylamide). The immunoprecipitated ERK was quantitated by immunoblotting.
FIG. 10
Rottlerin blocks neurite outgrowth in NGF-induced PC12 and estradiol-activated ΔRaf-1:ER cells. (A) PC12 cells untreated or pretreated with 5 μM rottlerin (Rott) and then exposed to 50 ng of NGF per ml for 4 days. Original magnification, ×100. (B) ΔRaf-1:ER cells untreated or pretreated with 5 μM rottlerin and then exposed to 1 μM estradiol for 1 day. Original magnification, ×40.
FIG. 11
PKCδ antisense oligonucleotides block neurite outgrowth in NGF-induced PC12 cells. (A) PC12 cells either untreated (CTRL) or pretreated with sense (PKCδ-S) or antisense (PKCδ-AS) ODNs as described in Materials and Methods and then stimulated with 50 ng of NGF per ml (+ NGF) for 5 days as indicated. Original magnification, ×100. (B) Immunoblots of PKCδ from cells treated as described for panel A. Cell lysates were collected and resolved by SDS-PAGE (10% polyacrylamide) and assayed for PKCδ expression by immunoblotting with anti-PKCδ antibody.
Similar articles
- Different protein kinase C isoforms determine growth factor specificity in neuronal cells.
Corbit KC, Soh JW, Yoshida K, Eves EM, Weinstein IB, Rosner MR. Corbit KC, et al. Mol Cell Biol. 2000 Aug;20(15):5392-403. doi: 10.1128/MCB.20.15.5392-5403.2000. Mol Cell Biol. 2000. PMID: 10891480 Free PMC article. - Requirement of p38 mitogen-activated protein kinase for neuronal differentiation in PC12 cells.
Morooka T, Nishida E. Morooka T, et al. J Biol Chem. 1998 Sep 18;273(38):24285-8. doi: 10.1074/jbc.273.38.24285. J Biol Chem. 1998. PMID: 9733710 - Role of MAP kinase in neurons.
Fukunaga K, Miyamoto E. Fukunaga K, et al. Mol Neurobiol. 1998 Feb;16(1):79-95. doi: 10.1007/BF02740604. Mol Neurobiol. 1998. PMID: 9554703 Review.
Cited by
- Regulation of MAPKs by growth factors and receptor tyrosine kinases.
Katz M, Amit I, Yarden Y. Katz M, et al. Biochim Biophys Acta. 2007 Aug;1773(8):1161-76. doi: 10.1016/j.bbamcr.2007.01.002. Epub 2007 Jan 10. Biochim Biophys Acta. 2007. PMID: 17306385 Free PMC article. Review. - An overview of brain-derived neurotrophic factor and implications for excitotoxic vulnerability in the hippocampus.
Murray PS, Holmes PV. Murray PS, et al. Int J Pept. 2011;2011:654085. doi: 10.1155/2011/654085. Epub 2011 Sep 28. Int J Pept. 2011. PMID: 21966294 Free PMC article. - Phosphorylation of serine 779 in fibroblast growth factor receptor 1 and 2 by protein kinase C(epsilon) regulates Ras/mitogen-activated protein kinase signaling and neuronal differentiation.
Lonic A, Powell JA, Kong Y, Thomas D, Holien JK, Truong N, Parker MW, Guthridge MA. Lonic A, et al. J Biol Chem. 2013 May 24;288(21):14874-85. doi: 10.1074/jbc.M112.421669. Epub 2013 Apr 5. J Biol Chem. 2013. PMID: 23564461 Free PMC article. - Protein kinase C-delta regulates thrombin-induced ICAM-1 gene expression in endothelial cells via activation of p38 mitogen-activated protein kinase.
Rahman A, Anwar KN, Uddin S, Xu N, Ye RD, Platanias LC, Malik AB. Rahman A, et al. Mol Cell Biol. 2001 Aug;21(16):5554-65. doi: 10.1128/MCB.21.16.5554-5565.2001. Mol Cell Biol. 2001. PMID: 11463837 Free PMC article. - Protein kinase C activity is necessary for estrogen-induced Erk phosphorylation in neocortical explants.
Sétáló G Jr, Singh M, Nethrapalli IS, Toran-Allerand CD. Sétáló G Jr, et al. Neurochem Res. 2005 Jun-Jul;30(6-7):779-90. doi: 10.1007/s11064-005-6871-y. Neurochem Res. 2005. PMID: 16187213
References
- Chao T-S O, Byron K L, Lee K-M, Villereal M, Rosner M R. Activation of MAP kinase by calcium-dependent and calcium-independent pathways. J Biol Chem. 1992;267:19876–19883. - PubMed
- Chou M M, Hou W, Johnson J, Graham L K, Lee M H, Chen C-S, Newton A C, Schaffhausen B S, Toker A. Regulation of protein kinase Cζ by PI 3-kinase and PDK-1. Curr Biol. 1998;8:1069–1077. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 GM007151/GM/NIGMS NIH HHS/United States
- CA46677/CA/NCI NIH HHS/United States
- 5 T32 GM 07151-24/GM/NIGMS NIH HHS/United States
- NS33858/NS/NINDS NIH HHS/United States
- R01 NS033858/NS/NINDS NIH HHS/United States
- G12 RR003037/RR/NCRR NIH HHS/United States
- R01 CA046677/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous