Simultaneous disruption of interleukin (IL)-4 and IL-13 defines individual roles in T helper cell type 2-mediated responses - PubMed (original) (raw)
Simultaneous disruption of interleukin (IL)-4 and IL-13 defines individual roles in T helper cell type 2-mediated responses
G J McKenzie et al. J Exp Med. 1999.
Abstract
Using a single vector targeting strategy, we have generated mice with a combined deficiency of interleukin (IL)-4 and IL-13 to clarify their roles in T helper type 2 (Th2) cell responses. Using immunological challenges normally characterized by a Th2-like response, we have compared the responses of the double-deficient mice with those generated by wild-type, IL-4-deficient, and IL-13-deficient mice. Using a pulmonary granuloma model, induced with Schistosoma mansoni eggs, we demonstrate that although eosinophil infiltration, immunoglobulin E, and IL-5 production are reduced in the IL-4-deficient mice and IL-13-deficient mice, they are abolished only in the combined absence of both cytokines. Furthermore, IL-4/13-deficient animals are severely impaired in their ability to expel the gastrointestinal nematode Nippostrongylus brasiliensis. Unexpectedly, N. brasiliensis-infected IL-4/13-deficient mice developed elevated IL-5 and eosinophilia, indicating that compensatory mechanisms exist for the expression of IL-5, although serum IgE remained undetectable. IL-4/13-deficient mice default to a Th1-like phenotype characterized by the expression of interferon gamma and the production of IgG2a and IgG2b. We conclude that IL-4 and IL-13 cooperate to initiate rapid Th2 cell-driven responses, and that although their functions overlap, they perform additive roles.
Figures
Figure 1
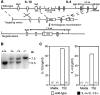
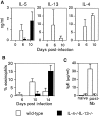
Simultaneous inactivation of the IL-4 and IL-13 genes by homologous recombination. (A) The structure of the two loci, the single targeting vector, and the predicted homologous recombination event are shown. Targeted disruption results in the deletion of the 15-kb region extending from within exon 3 of the IL-13 locus (white boxes) to a HindIII site in intron 3 of the IL-4 locus (gray boxes). H, HindIII; neo, neomycin resistance cassette; tk, thymidine kinase cassette. (B) Southern blotting of F2 tail genomic DNA. The indicated probe detects a 5.5-kb BglII fragment in the wild-type IL-4 gene and a 7.5-kb fragment as a result of the correct homologous recombination event between the IL-4 and IL-13 loci. (C) Analysis of IL-4 and IL-13 expression by CD4+ T cells. CD4+ T cells purified from two pooled spleens from two wild-type or two IL-4−/− IL-13−/− mice were stimulated under conditions promoting the expression of Th2 cytokines. Supernatants were analyzed by ELISA. Data are representative of three repeat experiments. ND, not detected.
Figure 2
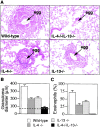
Analysis of pulmonary inflammatory response. (A) Morphological analysis of granuloma formation in wild-type, IL-4−/−, IL-13−/−, and IL-4−/−IL-13−/− mice. Cohorts of six mice were sensitized to S. mansoni eggs by intraperitoneal injection of 5,000 live eggs. After 15 d, these mice were injected intravenously with 5,000 eggs to induce synchronous pulmonary granuloma. Mice were killed 15 d later. Lung sections were stained with H&E. Original magnification: ×100. (B) Determination of granuloma diameters in immunized mice. Lung sections were stained with H&E, and at least 100 individual granulomas were measured per group. Dashed line indicates the mean diameter of an egg. (C) Eosinophil counts. After granuloma formation, the percent composition of eosinophils in the granulomas was determined from Giemsa-stained lung sections. Representative data from two repeat experiments (six to eight mice per group). Data are presented as means ± SD.
Figure 3
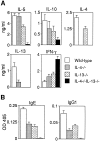
Cytokine and Ig responses to pulmonary challenge. (A) Cytokine responses from activated lymph node cells. Draining mediastinal lymph node cells were stimulated with soluble egg antigen, and supernatants were assayed for cytokines by ELISA. (B) Antigen-specific serum IgE and IgG1 after granuloma formation. Egg antigen–specific antibody isotypes were assayed by ELISA. Representative data from two repeat experiments using six to eight mice per group. Data are presented as means ± SD.
Figure 4
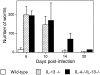
Analysis of infection with N. brasiliensis. Determination of worm burdens. Cohorts of five mice were infected with 400 viable third-stage N. brasiliensis larvae and killed at the times indicated to obtain intestinal worm counts. Representative data from two repeat experiments. Data are presented as means ± SD.
Figure 5
Analysis of cytokines, eosinophilia, and IgE in response to N. brasiliensis infection. (A) Cytokine expression from Con A–stimulated mesenteric lymph node cells from wild-type and IL-4−/−IL-13−/− animals after infection with N. brasiliensis. Lymph node cells (2 × 106 cells/ml) were cultured for 24 h in the presence of Con A (2 μg/ml). Supernatants were analyzed by cytokine ELISA. Representative data from two repeat experiments are shown. (B) Blood eosinophilia after N. brasiliensis infection. Peripheral blood was sampled at the times indicated, and the percentage of eosinophils was determined from blood smears stained with Giemsa. (C) Total serum IgE expression from wild-type and IL-4−/−IL-13−/− animals pre- and post- (day 14) infection with N. brasiliensis (Nb) assessed by ELISA. ND, not detected. Representative data from two repeat experiments using five mice per group. Data are presented as means ± SD.
Figure 6
Antigen-specific Ig response to OVA after immunization. (A) IL-13–deficient (○) and IL-4/13–deficient (▵) mice (shown against preimmune [□] and wild-type [▪] mice); (B) IL-4–deficient (•) mice (shown against preimmune [□] and C57BL/6 [♦] mice). Cohorts of four animals were immunized intraperitoneally with 100 μg of OVA adsorbed to alum with subsequent boost injections of OVA/alum after 10 and 20 d. Serum samples were assayed by ELISA for Ig isotypes. Representative data from two repeat experiments are shown.
Similar articles
- IL-13 is a key regulatory cytokine for Th2 cell-mediated pulmonary granuloma formation and IgE responses induced by Schistosoma mansoni eggs.
Chiaramonte MG, Schopf LR, Neben TY, Cheever AW, Donaldson DD, Wynn TA. Chiaramonte MG, et al. J Immunol. 1999 Jan 15;162(2):920-30. J Immunol. 1999. PMID: 9916716 - Differences between IL-4R alpha-deficient and IL-4-deficient mice reveal a role for IL-13 in the regulation of Th2 responses.
Barner M, Mohrs M, Brombacher F, Kopf M. Barner M, et al. Curr Biol. 1998 May 21;8(11):669-72. doi: 10.1016/s0960-9822(98)70256-8. Curr Biol. 1998. PMID: 9635196 - Interleukin-4-promoted T helper 2 responses enhance Nippostrongylus brasiliensis-induced pulmonary pathology.
Mearns H, Horsnell WG, Hoving JC, Dewals B, Cutler AJ, Kirstein F, Myburgh E, Arendse B, Brombacher F. Mearns H, et al. Infect Immun. 2008 Dec;76(12):5535-42. doi: 10.1128/IAI.00210-08. Epub 2008 Sep 22. Infect Immun. 2008. PMID: 18809669 Free PMC article. - Antagonizing the differentiation and functions of human T helper type 2 cells.
de Vries JE, Carballido JM, Sornasse T, Yssel H. de Vries JE, et al. Curr Opin Immunol. 1995 Dec;7(6):771-8. doi: 10.1016/0952-7915(95)80046-8. Curr Opin Immunol. 1995. PMID: 8679118 Review. - Immune responses of IL-4, IL-5, IL-6 deficient mice.
Kopf M, Le Gros G, Coyle AJ, Kosco-Vilbois M, Brombacher F. Kopf M, et al. Immunol Rev. 1995 Dec;148:45-69. doi: 10.1111/j.1600-065x.1995.tb00093.x. Immunol Rev. 1995. PMID: 8825282 Review. No abstract available.
Cited by
- Inverse Correlation of Th2-Specific Cytokines with Hepatic Egg Burden in _S. mansoni_-Infected Hamsters.
Russ L, von Bülow V, Wrobel S, Stettler F, Schramm G, Falcone FH, Grevelding CG, Roderfeld M, Roeb E. Russ L, et al. Cells. 2024 Sep 20;13(18):1579. doi: 10.3390/cells13181579. Cells. 2024. PMID: 39329761 Free PMC article. - Adjuvant-independent airway sensitization and infection mouse models leading to allergic asthma.
Radhouani M, Starkl P. Radhouani M, et al. Front Allergy. 2024 Aug 2;5:1423938. doi: 10.3389/falgy.2024.1423938. eCollection 2024. Front Allergy. 2024. PMID: 39157265 Free PMC article. Review. - Heligmosomoides bakeri and Toxoplasma gondii co-infection leads to increased mortality associated with changes in immune resistance in the lymphoid compartment and disease pathology.
Szabo EK, Bowhay C, Forrester E, Liu H, Dong B, Coria AL, Perera S, Fung B, Badawadagi N, Gaio C, Bailey K, Ritz M, Bowron J, Ariyaratne A, Finney CAM. Szabo EK, et al. PLoS One. 2024 Jul 1;19(7):e0292408. doi: 10.1371/journal.pone.0292408. eCollection 2024. PLoS One. 2024. PMID: 38950025 Free PMC article. - Vernal Keratoconjunctivitis: Immunopathological Insights and Therapeutic Applications of Immunomodulators.
Hehar NK, Chigbu DI. Hehar NK, et al. Life (Basel). 2024 Mar 9;14(3):361. doi: 10.3390/life14030361. Life (Basel). 2024. PMID: 38541686 Free PMC article. Review. - Cytokines in Allergic Conjunctivitis: Unraveling Their Pathophysiological Roles.
Chigbu DI, Karbach NJ, Abu SL, Hehar NK. Chigbu DI, et al. Life (Basel). 2024 Mar 7;14(3):350. doi: 10.3390/life14030350. Life (Basel). 2024. PMID: 38541674 Free PMC article. Review.
References
- Kuhn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4-deficient mice. Science. 1991;254:707–710. - PubMed
- Kopf M, Le Gros G, Bachmann M, Lamers C, Bluethmann H, Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362:245–248. - PubMed
- Barner M, Mohrs M, Brombacher F, Kopf M. Differences between IL-4Rα-deficient and IL-4-deficient mice reveal a role for IL-13 in the regulation of Th2 responses. Curr Biol. 1998;8:669–672. - PubMed
- Shimoda K, van Deursen J, Sangster M, Sarawar S, Carson R, Tripp R, Chu C, Quelle F, Nosaka T, Vignali D, et al. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–633. - PubMed
- Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, Nakanishi N, Yoshida N, Kishimoto T, Akira S. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380:627–630. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases