Interferon-gamma (IFN-gamma)- and tumour necrosis factor (TNF)-induced nitric oxide as toxic effector molecule in chronic dextran sulphate sodium (DSS)-induced colitis in mice - PubMed (original) (raw)
Interferon-gamma (IFN-gamma)- and tumour necrosis factor (TNF)-induced nitric oxide as toxic effector molecule in chronic dextran sulphate sodium (DSS)-induced colitis in mice
F Obermeier et al. Clin Exp Immunol. 1999 May.
Abstract
Excess nitric oxide formation caused by the activity of the inducible nitric oxide synthase has been implicated as a toxic effector molecule in the pathogenesis of experimental colitis and inflammatory bowel disease. It was therefore investigated whether inhibition of this synthase or the cytokines TNF and IFN-gamma, inducers of nitric oxide synthase, had effects on chronic colitis in mice. Chronic colitis was induced in mice by repeated feeding of DSS. Cytokines were neutralized by treatment with MoAbs and nitric oxide synthase was inhibited by aminoguanidine. The degree of colonic inflammation was assessed by a histological score and colon length. Aminoguanidine treatment reduced nitric oxide activity by 60% (P = 0. 0004), the histological score by 31% (P = 0.005) and increased colon length by 1.4 cm (P = 0.002). Neutralization of TNF and IFN-gamma resulted in increased colon length (0.7 cm, P = 0.07 and 0.8 cm, P = 0.03), improved histological score (19%, P = 0.045 and 25%, P = 0. 013), and reduced nitric oxide activity (31%, P = 0.07 and 54%, P = 0.004) compared with controls. The combination of anti-cytokine treatments had additive effects. TNF and IFN-gamma are involved in perpetuation of chronic DSS-induced colitis, and induction of excessive nitric oxide activity could be their common effector mechanism.
Figures
Fig. 1
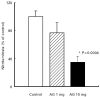
Effect of aminoguanidine (AG) on colonic NO activity. Mice with chronic DSS-induced colitis received either PBS (control), 1 mg or 10 mg per mouse AG from day 1 to day 5 of the experiment. Nitrite/nitrate contents were determined as described in Materials and Methods. Each group consisted of 9–10 mice. The result is representative of three separate experiments. Bars represent mean ± s.e.m. *Significantly different from control group.
Fig. 2
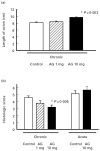
Effect of aminoguanidine (AG) on inflammatory parameters in chronic and acute colitis. Mice with chronic DSS-induced colitis received either PBS (control), 1 mg or 10 mg per mouse AG from day 1 to day 5 of the experiment or in acute colitis 10 mg AG per mouse per day or PBS (control) from day 0–7 of the induction phase. Colonic length (a) and histological score (b) were determined as described in Materials and Methods. In experiments each group consisted of 9–10 mice. The result is representative of three separate experiments. Bars represent mean ± s.e.m. *Significantly different from control groups.
Fig. 3
Kinetics of body weight of mice with acute colitis. During induction of acute colitis (days 0–7) mice were treated daily with aminoguanidine (AG) at 10 mg per mouse (▪) or with PBS (control) (•). Body weight of individual mice was monitored daily. In experiments each group consisted of five mice. The result is representative of three separate experiments. Data points represent mean values ± s.d.
Fig. 4
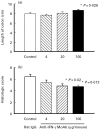
Effect of IFN-γ neutralization on inflammatory parameters in chronic colitis. Mice with chronic DSS-induced colitis received either rat IgG (control), 4 μg, 20 μg or 100 μg of neutralizing IFN-γ MoAb from day 1 to day 5 of the experiment. Colonic length (a) and histological score (b) were determined as described in Materials and Methods. Each group consisted of 9–10 mice. The result is representative of three separate experiments. Bars represent mean ± s.e.m. *Significantly different from control groups.
Fig. 5
Effect of neutralization of IFN-γ on colonic nitric oxide (NO) activity. Mice with chronic DSS-induced colitis received either rat IgG (control), 4 μg, 20 μg or 100 μg of neutralizing IFN-γ MoAb from day 1 to day 5 of the experiment. Nitrite/nitrate concentrations were determined as described in Materials and Methods. Each group consisted of 9–10 mice. The result is representative of three separate experiments. Bars represent mean ± s.e.m. *Significantly different from control group.
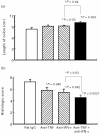
Fig. 6
Effect of neutralization of TNF and IFN-γ in combination on parameters of colitis. Mice with chronic DSS-induced colitis received either rat IgG (control), 100 μg of neutralizing IFN-γ MoAb, 100 μg of neutralizing TNF MoAb or a combination of both MoAbs from day 1 to day 5 of the experiment. Colonic length (a) and histological score (b) were determined as described in Materials and Methods. Each group consisted of 10 mice. The result is representative of three separate experiments. Bars represent mean ± s.e.m. *Significantly different from control groups unless otherwise indicated.
Fig. 7
Effect of neutralization of TNF and IFN-γ in combination on nitric oxide (NO) activity. Mice with chronic DSS-induced colitis received either rat IgG (control), 100 μg of neutralizing IFN-γ MoAb, 100 μg of neutralizing TNF MoAb or a combination of both MoAbs from day 1 to day 5 of the experiment. Nitrite/nitrate concentrations were determined as described in Materials and Methods. Each group consisted of 10 mice. The result is representative of two separate experiments. Bars represent mean ± s.e.m. *Significantly different from control groups unless otherwise indicated.
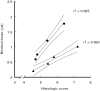
Fig. 8
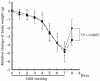
Correlation of the histological score and nitrite/nitrate concentrations of colonic tissue. The histological scores (abscissa) and nitrite/nitrate (ordinate) concentrations measured in the anti-IFN-γ dose response experiment (•) and in the anti-IFN-γ/anti-TNF combination experiment (▴) were correlated. Results were taken from experiments presented in Fig. 4b and Fig. 5 (anti-IFN-γ dose response) and in Fig. 6b and Fig. 7 (anti-IFN-γ/anti-TNF combination). A confidence interval of 95% is given. _r_2 were derived from linear regression analysis.
Similar articles
- alpha-Phenyl-N-tert-butylnitrone provides protection from dextran sulfate sodium-induced colitis in mice.
Naito Y, Takagi T, Ishikawa T, Handa O, Matsumoto N, Yagi N, Matsuyama K, Yoshida N, Yoshikawa T, Kotake Y. Naito Y, et al. Antioxid Redox Signal. 2002 Feb;4(1):195-206. doi: 10.1089/152308602753625951. Antioxid Redox Signal. 2002. PMID: 11970853 - Enhanced intestinal inflammation induced by dextran sulfate sodium in tumor necrosis factor-alpha deficient mice.
Naito Y, Takagi T, Handa O, Ishikawa T, Nakagawa S, Yamaguchi T, Yoshida N, Minami M, Kita M, Imanishi J, Yoshikawa T. Naito Y, et al. J Gastroenterol Hepatol. 2003 May;18(5):560-9. doi: 10.1046/j.1440-1746.2003.03034.x. J Gastroenterol Hepatol. 2003. PMID: 12702049 - The detrimental effect of nitric oxide on tissue is associated with inflammatory events in the vascular endothelium and neutrophils in mice with dextran sodium sulfate-induced colitis.
Yasukawa K, Tokuda H, Tun X, Utsumi H, Yamada K. Yasukawa K, et al. Free Radic Res. 2012 Dec;46(12):1427-36. doi: 10.3109/10715762.2012.732698. Epub 2012 Oct 9. Free Radic Res. 2012. PMID: 22998024 - The role of tumour necrosis factor, interleukin 6, interferon-gamma and inducible nitric oxide synthase in the development and pathology of the nervous system.
Muñoz-Fernández MA, Fresno M. Muñoz-Fernández MA, et al. Prog Neurobiol. 1998 Oct;56(3):307-40. doi: 10.1016/s0301-0082(98)00045-8. Prog Neurobiol. 1998. PMID: 9770242 Review. - Review article: the potential role of nitric oxide in chronic inflammatory bowel disorders.
Perner A, Rask-Madsen J. Perner A, et al. Aliment Pharmacol Ther. 1999 Feb;13(2):135-44. doi: 10.1046/j.1365-2036.1999.00453.x. Aliment Pharmacol Ther. 1999. PMID: 10102942 Review.
Cited by
- Dietary Supplement of Amomum villosum Lour. Polysaccharide Attenuates Ulcerative Colitis in BALB/c Mice.
Luo D, Zeng J, Guan J, Xu Y, Jia RB, Chen J, Jiang G, Zhou C. Luo D, et al. Foods. 2022 Nov 21;11(22):3737. doi: 10.3390/foods11223737. Foods. 2022. PMID: 36429334 Free PMC article. - Smad4-deficient T cells promote colitis-associated colon cancer via an IFN-γ-dependent suppression of 15-hydroxyprostaglandin dehydrogenase.
Choi SH, Huang AY, Letterio JJ, Kim BG. Choi SH, et al. Front Immunol. 2022 Aug 15;13:932412. doi: 10.3389/fimmu.2022.932412. eCollection 2022. Front Immunol. 2022. PMID: 36045676 Free PMC article. - Plasticity of Paneth cells and their ability to regulate intestinal stem cells.
Mei X, Gu M, Li M. Mei X, et al. Stem Cell Res Ther. 2020 Aug 12;11(1):349. doi: 10.1186/s13287-020-01857-7. Stem Cell Res Ther. 2020. PMID: 32787930 Free PMC article. Review. - Galectin-3 Regulates Indoleamine-2,3-dioxygenase-Dependent Cross-Talk between Colon-Infiltrating Dendritic Cells and T Regulatory Cells and May Represent a Valuable Biomarker for Monitoring the Progression of Ulcerative Colitis.
Volarevic V, Zdravkovic N, Harrell CR, Arsenijevic N, Fellabaum C, Djonov V, Lukic ML, Simovic Markovic B. Volarevic V, et al. Cells. 2019 Jul 12;8(7):709. doi: 10.3390/cells8070709. Cells. 2019. PMID: 31336879 Free PMC article. - OGR1 (GPR68) and TDAG8 (GPR65) Have Antagonistic Effects in Models of Colonic Inflammation.
Perren L, Busch M, Schuler C, Ruiz PA, Foti F, Weibel N, de Vallière C, Morsy Y, Seuwen K, Hausmann M, Rogler G. Perren L, et al. Int J Mol Sci. 2023 Oct 3;24(19):14855. doi: 10.3390/ijms241914855. Int J Mol Sci. 2023. PMID: 37834303 Free PMC article.
References
- Reinecker HC, Steffen M, Witthoeft T, Pflueger I, Schreiber S, MacDermott RP, Raedler A. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1993;94:174–81. - PMC - PubMed
- Murata Y, Ishiguro Y, Itoh J, Munakata A, Yoshida Y. The role of proinflammatory and immunoregulatory cytokines in the pathogenesis of ulcerative colitis. J Gastroenterol. 1995;30(Suppl. 8):56–60. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources