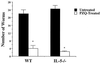Interleukin 5 (IL-5) is not required for expression of a Th2 response or host resistance mechanisms during murine schistosomiasis mansoni but does play a role in development of IL-4-producing non-T, non-B cells - PubMed (original) (raw)
Interleukin 5 (IL-5) is not required for expression of a Th2 response or host resistance mechanisms during murine schistosomiasis mansoni but does play a role in development of IL-4-producing non-T, non-B cells
L R Brunet et al. Infect Immun. 1999 Jun.
Abstract
During schistosomiasis, interleukin-5 (IL-5)-dependent eosinophil responses have been implicated in immunopathology, resistance to superinfection, synergistic interactions with chemotherapeutic agents, and the inductive phase of the egg-induced Th2 response. We examined these issues in IL-5-deficient (IL-5(-/-)) mice. IL-5(-/-) and wild-type (WT) mice were indistinguishable in terms of susceptibility to primary infections and the ability to resist secondary infections. Moreover, hepatic pathology was similar in both strains apart from a relative lack of eosinophils and, during chronic infection, a significantly larger mast cell component in the granulomas of IL-5(-/-) mice. Splenocyte cytokine production in response to soluble egg antigen (SEA) or anti-CD3 revealed no significant differences except for heightened tumor necrosis factor alpha production by cells from chronically infected IL-5(-/-) mice compared to WT animals. In contrast, ionomycin-stimulated non-B, non-T (NBNT) cells from IL-5(-/-) mice produced significantly smaller IL-4 amounts than did NBNT cells from WT animals. This difference was not apparent following plate-bound anti-immunoglobulin E or SEA stimulation. The absence of IL-5 failed to affect the induction of Th2 responses in naive mice. Peritoneal exudate cells recovered from egg-injected IL-5(-/-) or WT mice produced equivalent levels of IL-4 following restimulation with SEA or anti-CD3.
Figures
FIG. 1
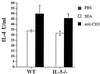
Comparison of IL-4 cytokine levels from PEC of WT and IL-5−/− mice (three animals per group) injected 10 days earlier with either S. mansoni eggs or PBS. PEC were pooled and cultured for 72 h in duplicate wells in the presence of anti-IL-4R either alone, with SEA, or with plate-bound anti-CD3. Data are from one experiment; the experiment was repeated three times with similar results. Data are expressed as means ± standard errors.
FIG. 2
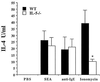
Comparison of IL-4 levels produced by NBNT cells of WT and IL-5−/− mice (at least three animals per group) infected with S. mansoni (8 weeks postinfection). NBNT cells were cultured for 24 h with PBS, SEA, plate-bound anti-IgE, or ionomycin. Results are from one experiment and are representative of data from three separate experiments. Data are expressed as means ± standard errors. An asterisk indicates P < 0.05 compared with WT animals.
FIG. 3
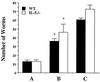
Comparison of resistance to superinfection in WT and IL-5−/− mice. Worm burdens in infected WT and IL-5−/− mice were determined after perfusion. Mice received a primary infection (group A; four WT and six IL-5−/− mice) primary and secondary infections (group B; five WT and five IL-5−/− mice), or a secondary infection (group C; six WT and six IL-5−/− mice). Resistance to reinfection was observed in both WT (61.4% ± 4.1%) and IL-5−/− (53.6% ± 10.2%) mice in group B. Data are expressed as means ± standard errors. An asterisk indicates P < 0.05 compared with groups receiving a challenge infection alone.
FIG. 4
Effect of praziquantel on worm burden in IL-5−/− and WT mice. Mice (three animals per group) were treated with praziquantel in carrier or with carrier alone. Data are expressed as means ± standard errors. Results are representative of two separate experiments. An asterisk indicates P < 0.05 compared with untreated groups.
Similar articles
- Th2-type granuloma development in acute murine schistosomiasis is only partly dependent on CD4+ T cells as the source of IL-4.
Metwali A, de Andres B, Blum A, Elliott D, Li J, Qadir K, Sandor M, Weinstock J. Metwali A, et al. Eur J Immunol. 2002 May;32(5):1242-52. doi: 10.1002/1521-4141(200205)32:5<1242::AID-IMMU1242>3.0.CO;2-7. Eur J Immunol. 2002. PMID: 11981811 - Fc epsilonRI-deficient mice infected with Schistosoma mansoni mount normal Th2-type responses while displaying enhanced liver pathology.
Jankovic D, Kullberg MC, Dombrowicz D, Barbieri S, Caspar P, Wynn TA, Paul WE, Cheever AW, Kinet JP, Sher A. Jankovic D, et al. J Immunol. 1997 Aug 15;159(4):1868-75. J Immunol. 1997. PMID: 9257851 - T cell-derived IL-3 induces the production of IL-4 by non-B, non-T cells to amplify the Th2-cytokine response to a non-parasite antigen in Schistosoma mansoni-infected mice.
Kullberg MC, Berzofsky JA, Jankovic DL, Barbieri S, Williams ME, Perlmann P, Sher A, Troye-Blomberg M. Kullberg MC, et al. J Immunol. 1996 Feb 15;156(4):1482-9. J Immunol. 1996. PMID: 8568251 - In infection with Schistosoma mansoni, B cells are required for T helper type 2 cell responses but not for granuloma formation.
Hernandez HJ, Wang Y, Stadecker MJ. Hernandez HJ, et al. J Immunol. 1997 May 15;158(10):4832-7. J Immunol. 1997. PMID: 9144498 - Role of eosinophils in protective immunity against secondary nematode infections.
Yasuda K, Kuroda E. Yasuda K, et al. Immunol Med. 2019 Dec;42(4):148-155. doi: 10.1080/25785826.2019.1697135. Epub 2019 Dec 3. Immunol Med. 2019. PMID: 31794348 Review.
Cited by
- Aberrant in vivo T helper type 2 cell response and impaired eosinophil recruitment in CC chemokine receptor 8 knockout mice.
Chensue SW, Lukacs NW, Yang TY, Shang X, Frait KA, Kunkel SL, Kung T, Wiekowski MT, Hedrick JA, Cook DN, Zingoni A, Narula SK, Zlotnik A, Barrat FJ, O'Garra A, Napolitano M, Lira SA. Chensue SW, et al. J Exp Med. 2001 Mar 5;193(5):573-84. doi: 10.1084/jem.193.5.573. J Exp Med. 2001. PMID: 11238588 Free PMC article. - Contribution of parasite and host genotype to immunopathology of schistosome infections.
Jutzeler KS, Le Clec'h W, Chevalier FD, Anderson TJC. Jutzeler KS, et al. Parasit Vectors. 2024 May 7;17(1):203. doi: 10.1186/s13071-024-06286-6. Parasit Vectors. 2024. PMID: 38711063 Free PMC article. - In vivo imaging of tissue eosinophilia and eosinopoietic responses to schistosome worms and eggs.
Davies SJ, Smith SJ, Lim KC, Zhang H, Purchio AF, McKerrow JH, West DB. Davies SJ, et al. Int J Parasitol. 2005 Jul;35(8):851-9. doi: 10.1016/j.ijpara.2005.02.017. Int J Parasitol. 2005. PMID: 15950229 Free PMC article. - Schistosoma mansoni infection in eosinophil lineage-ablated mice.
Swartz JM, Dyer KD, Cheever AW, Ramalingam T, Pesnicak L, Domachowske JB, Lee JJ, Lee NA, Foster PS, Wynn TA, Rosenberg HF. Swartz JM, et al. Blood. 2006 Oct 1;108(7):2420-7. doi: 10.1182/blood-2006-04-015933. Epub 2006 Jun 13. Blood. 2006. PMID: 16772607 Free PMC article. - Do helminths cause epilepsy?
Wagner RG, Newton CR. Wagner RG, et al. Parasite Immunol. 2009 Nov;31(11):697-705. doi: 10.1111/j.1365-3024.2009.01128.x. Parasite Immunol. 2009. PMID: 19825109 Free PMC article. Review.
References
- Brindley P, Sher A. The chemotherapeutic effect of praziquantel against Schistosoma mansoni is dependent on host antibody response. J Immunol. 1987;139:215–220. - PubMed
- Brindley P, Sher A. Immunological involvement in the efficacy of praziquantel. Exp Parasitol. 1990;71:245–248. - PubMed
- Butterworth A E, Sturrock R F, Houba V, Mahmoud A A F, Sher A, Rees P H. Eosinophils as mediators of antibody dependent damage to schistosomula. Nature. 1975;256:727–729. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 AI009227/AI/NIAID NIH HHS/United States
- R56 AI032573/AI/NIAID NIH HHS/United States
- F32 AI009512/AI/NIAID NIH HHS/United States
- R01 AI032573/AI/NIAID NIH HHS/United States
- AI32573/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases