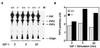PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B signaling pathway - PubMed (original) (raw)
PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B signaling pathway
H Sun et al. Proc Natl Acad Sci U S A. 1999.
Abstract
To investigate the molecular basis of PTEN-mediated tumor suppression, we introduced a null mutation into the mouse Pten gene by homologous recombination in embryonic stem (ES) cells. Pten-/- ES cells exhibited an increased growth rate and proliferated even in the absence of serum. ES cells lacking PTEN function also displayed advanced entry into S phase. This accelerated G1/S transition was accompanied by down-regulation of p27(KIP1), a major inhibitor for G1 cyclin-dependent kinases. Inactivation of PTEN in ES cells and in embryonic fibroblasts resulted in elevated levels of phosphatidylinositol 3,4,5,-trisphosphate, a product of phosphatidylinositol 3 kinase. Consequently, PTEN deficiency led to dosage-dependent increases in phosphorylation and activation of Akt/protein kinase B, a well-characterized target of the phosphatidylinositol 3 kinase signaling pathway. Akt activation increased Bad phosphorylation and promoted Pten-/- cell survival. Our studies suggest that PTEN regulates the phosphatidylinositol 3,4, 5,-trisphosphate and Akt signaling pathway and consequently modulates two critical cellular processes: cell cycle progression and cell survival.
Figures
Figure 1
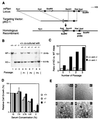
Inactivation of the mouse Pten gene and the growth properties of _Pten_−/− ES cells. (A) A restriction map of the genomic region containing the Pten gene is shown at the top, with exons depicted. The targeting vector pKO-1 is shown in the middle. A restriction map of the predicted recombinant harboring the deleted allele is shown at the bottom. (B) Southern blot analysis. Lanes 1–3: DNA from Pten+/+ (+/+, lane 1), heterozygous (+/−, lane 2), and homozygous (−/−, lane 3) ES cell cultures. Lanes 4–9: DNA from cocultured ES cells. An equal number (4 × 105) of +/+ cells were cocultured with +/− cells in 33-mm dish (lanes 4, 6, and 8). Alternatively, an equal number of +/+ cells were cocultured with −/− cells (lanes 5, 7, and 9). DNA isolated from the indicated cultures were analyzed after one, two, or three passages. After _Eco_RV digestion, 23-kb and 8.5-kb bands, corresponding to the WT allele or the targeted allele (KO), respectively, could be detected by using an external probe. (C) Quantification of the amount of radioactivity in the hybridized restriction fragment corresponding to the WT allele (23 kb) or the Pten deletion allele (KO, 8.5 kb). The relative hybridization intensity of KO versus WT band in cocultures is presented. (D) ES cells (1 × 105 cells/well in 24-well plate) were grown in media containing the indicated serum concentrations. Four days later, cells were counted. The relative cell growth was calculated by using cell numbers in 15% serum condition as 100%. Each value represents the average (±SD) obtained from a duplicate set of experiments. (E) WT (+/+, a), heterozygous (+/−, b) and two independent homozygous (−/−, c and d) ES cell lines were grown in serum-free medium for 4 days. Although no ES colonies, except the background feeder cell layers, were seen in the WT and heterozygous ES cultures (a and b, respectively), visible ES cell colonies (indicated by the arrowheads) were present in homozygous ES cultures (c and d). Scale bar: 200 μM.
Figure 2
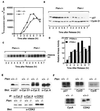
Cell cycle progression and p27KIP1 levels in synchronized and asynchronous Pten+/+ and _Pten_−/− cells. (A) [3H]thymidine incorporation after release from the colcemid block. For each time point, cells (1 × 105) were pulse-labeled with [3H]thymidine (1 μCi/ml) for 1 hr before harvesting. [3H]thymidine incorporation was measured. Each value represents the average (+SD) obtained from duplicate samples. (B) Western blot analysis of p27KIP1 and cyclin D1 levels after release from colcemid block. Approximately 1 × 106 cells were seeded for each time point, and cell lysates (50 μg each) were examined by Western blot analysis with anti-p27 or anti-cyclin D1 antibody, respectively. (C and D) Histone H1 kinase activity assay. Cells were synchronized as described in B. Cyclin E/CDK2 complex was immunoprecipitated with anti-cyclin E antibody from cell lysates (300 μg each) and assayed for in vitro kinase activity by using histone H1 as substrate and 32p-[γ]-ATP. The relative kinase activity was obtained after quantification of the 32P-label incorporated into histone H1 by PhosphorImager. (E) Western blot analysis of various cell cycle regulators. Cell lysates from log-phase growing Pten+/+ or _Pten_−/− cells (50 μg each) were analyzed by Western blot analysis with antibodies specific for p27KIP1 or cyclin D1 (Upper). To examine the level of cyclin E or cyclin A, cell lysates (2 mg each) were immunoprecipitated with anti-cyclin E or anti-cyclin A antibodies, respectively, followed by Western blot analysis with the corresponding antibodies (Upper). (Lower) Cell lysates (2 mg each) were immunoprecipitated with antibodies for cyclin E, cyclin A, or CDK2, and analyzed with Western blot analysis with anti-CDK2 antibody. (F) Northern blot analysis. Total RNA (5 μg each) harvested from log-phase growing Pten+/+ or _Pten_−/− cells were subjected to Northern blot analysis using p27, cyclin D, cyclin E, cyclin A, or CDK2 cDNA probe, respectively.
Figure 3
PIP3 accumulation in Pten+/+ and _Pten_−/− cells. (A) PIP3 levels in Pten+/+ and _Pten_−/− ES cells after IGF-I stimulation. Cells were starved in a serum-free medium for 16 hr, and then labeled with [32P]orthophosphate (0.5 mCi/ml) for 2 hr. Cells then were stimulated by IGF-I (1 μg/ml) for 2, 5, or 20 min before harvesting. Phospholipids were extracted and analyzed on a TLC plate. Assignment of PIP, PIP2, and PIP3 was done according to in vitro 32P-labeled phosphoinositides standards (see Materials and Methods). In lane M, [32P]-labeled PIP3 is shown as a marker. (B) Quantitation of PIP3 levels in Pten+/+ and _Pten_−/− ES cells after IGF-I stimulation. The amount of radioactivity corresponding to PIP3 was measured with a PhosphorImager and presented as an arbitrary unit.
Figure 4
Phosphorylation status of Akt, PI3 kinase, MAPK, and FAK, and the levels of p27 in Pten+/+, Pten+/−, or _Pten_−/− ES cells. (A) Phosphorylation status of Akt after IGF-I stimulation. Pten+/+ and _Pten_−/− ES cells were passed twice without feeders to reduced background. Cells were serum-starved for 34 hr, then stimulated by IGF-I (1 μg/ml) for indicated time periods. Cell lysates (25 μg each) were examined by Western blot analysis with antibodies against phospho-Akt (serine-473) or Akt, respectively. (B) Phosphorylation status of Akt in actively growing ES cells. Log-phase growing Pten+/+, Pten+/−, or _Pten_−/− ES cells were harvested, and the cell lysates were analyzed with antibodies against phospho-Akt or Akt, respectively. (C) Akt, p27KIP1, and cyclin D1 levels in cells from different proliferation states. Cells were harvested from either log-phase cultures (Left) or confluent cultures (Right). Cell lysates (50 μg each) were examined by Western blot analysis with antibody specific for phospho-Akt, p27, or cyclin D1, respectively. (D) Phosphorylation status of PI3 kinase, MAPK, and FAK. Cell lysates were prepared from log-phase growing cells. To determine the phosphorylation status of the p85 subunit of PI3 kinase and FAK, cell lysates (500 μg each) were immunoprecipitated with antiphosphotyrosine antibody 4G10 followed by Western blot analysis with anti-p85 or anti-FAK antibody, respectively. To detect phosphorylated p42 and p44 MAPK, cell lysates (50 μg each) were examined by Western blots analysis with an antibody against phospho-MAPK. As a control, a duplicate filter was analyzed in parallel with an antibody for p42 MAPK.
Figure 5
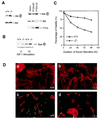
Increased phosphorylation of Akt and Bad promotes Pten_−/− MEF cells survival. (A, Left) MEF cell lysates were prepared and subjected to Western blot analyses as described in the legend of Fig. 4_B. (A, Right) _Pten_−/− MEF cells were infected with retroviruses carrying empty vector, the WT PTEN, or the PTEN CS mutant. Cells were harvested 48 hr postinfection. Cell lysates (50 μg each) were subjected to Western blot analysis with antibodies specific for phospho-Akt or Akt, respectively. A duplicate filter also was analyzed with anti-PTEN antibody. (B) MEF cells were serum-starved for 16 hr, then labeled with [32P]orthrophosphate for 4 hr. Cells then were stimulated with IGF-I (1 μg/ml) for 10 min before harvest. Cell lysates were subjected to immunoprecipitation with anti-Bad antibody, and the immunoprecipitates were analyzed by SDS/PAGE and autoradiography. (C) Propidium iodide staining. Pten+/+ or _Pten_−/− MEF cells were seeded in serum-free medium. At the indicated time, cells (both adherent and in suspension) were collected and stained with isotonic propidium iodide solution. Percentage of cell viability, determined by using fluorescence-activated cell sorting analysis, is presented. (D) TUNEL assay. Log-phase Pten+/+ (a and c) and _Pten_−/− (b and d) MEF cells were grown with (a and b) or without (c and d) serum for 72 hr. Cells were stained with TUNEL reaction mix (green) and counterstained with rhodamine-phalloidin (red). Apoptotic cells were indicated by positive staining with both TUNEL reaction mix and pholloidin dye (yellow).
Similar articles
- PTEN/MMAC1/TEP1 suppresses the tumorigenicity and induces G1 cell cycle arrest in human glioblastoma cells.
Li DM, Sun H. Li DM, et al. Proc Natl Acad Sci U S A. 1998 Dec 22;95(26):15406-11. doi: 10.1073/pnas.95.26.15406. Proc Natl Acad Sci U S A. 1998. PMID: 9860981 Free PMC article. - Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway.
Ramaswamy S, Nakamura N, Vazquez F, Batt DB, Perera S, Roberts TM, Sellers WR. Ramaswamy S, et al. Proc Natl Acad Sci U S A. 1999 Mar 2;96(5):2110-5. doi: 10.1073/pnas.96.5.2110. Proc Natl Acad Sci U S A. 1999. PMID: 10051603 Free PMC article. - PTEN regulates the ubiquitin-dependent degradation of the CDK inhibitor p27(KIP1) through the ubiquitin E3 ligase SCF(SKP2).
Mamillapalli R, Gavrilova N, Mihaylova VT, Tsvetkov LM, Wu H, Zhang H, Sun H. Mamillapalli R, et al. Curr Biol. 2001 Feb 20;11(4):263-7. doi: 10.1016/s0960-9822(01)00065-3. Curr Biol. 2001. PMID: 11250155 - [PTEN expression in endometrial cancer and the prognosis].
Kanamori Y, Uegaki K, Kigawa J, Terakawa N. Kanamori Y, et al. Nihon Rinsho. 2004 Oct;62 Suppl 10:406-9. Nihon Rinsho. 2004. PMID: 15535277 Review. Japanese. No abstract available. - Pten signaling in gliomas.
Knobbe CB, Merlo A, Reifenberger G. Knobbe CB, et al. Neuro Oncol. 2002 Jul;4(3):196-211. Neuro Oncol. 2002. PMID: 12084351 Free PMC article. Review.
Cited by
- Phosphatidylinositol-3 kinase signaling controls survival and stemness of hematopoietic stem and progenitor cells.
Blokzijl-Franke S, Ponsioen B, Schulte-Merker S, Herbomel P, Kissa K, Choorapoikayil S, den Hertog J. Blokzijl-Franke S, et al. Oncogene. 2021 Apr;40(15):2741-2755. doi: 10.1038/s41388-021-01733-5. Epub 2021 Mar 13. Oncogene. 2021. PMID: 33714985 Free PMC article. - Long non-coding RNA-ZNF281 upregulates PTEN expression via downregulation of microRNA-221 in non-small cell lung cancer.
Lu X, Yin B, Wang X, Wang F, Li Y, Wang N, Yang X, Jiang W. Lu X, et al. Oncol Lett. 2020 Sep;20(3):2962-2968. doi: 10.3892/ol.2020.11821. Epub 2020 Jul 7. Oncol Lett. 2020. PMID: 32782613 Free PMC article. - Activation of PDK-1 maintains mouse embryonic stem cell self-renewal in a PKB-dependent manner.
Ling LS, Voskas D, Woodgett JR. Ling LS, et al. Oncogene. 2013 Nov 21;32(47):5397-408. doi: 10.1038/onc.2013.44. Epub 2013 Mar 4. Oncogene. 2013. PMID: 23455320 Free PMC article. - Rescue of glandular dysmorphogenesis in PTEN-deficient colorectal cancer epithelium by PPARγ-targeted therapy.
Jagan I, Fatehullah A, Deevi RK, Bingham V, Campbell FC. Jagan I, et al. Oncogene. 2013 Mar 7;32(10):1305-15. doi: 10.1038/onc.2012.140. Epub 2012 Apr 30. Oncogene. 2013. PMID: 22543585 Free PMC article. - LncRNA HOTAIR impairs the prognosis of papillary thyroid cancer via regulating cellular malignancy and epigenetically suppressing DLX1.
Kuo FC, Wang YT, Liu CH, Li YF, Lu CH, Su SC, Liu JS, Li PF, Huang CL, Ho LJ, Lin CM, Lee CH. Kuo FC, et al. Cancer Cell Int. 2022 Dec 9;22(1):396. doi: 10.1186/s12935-022-02817-2. Cancer Cell Int. 2022. PMID: 36494673 Free PMC article.
References
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang S I, Puc J, Miliaresis C, Rodgers L, McCombie R, et al. Science. 1997;275:1943–1947. - PubMed
- Steck P A, Pershouse M A, Jasser S A, Lin H, Yung W K A, Ligon A H, Langford L A, Baumgard M L, Hattier T, Davis T, et al. Nat Genet. 1997;15:356–363. - PubMed
- Li D-M, Sun H. Cancer Res. 1997;57:2124–2129. - PubMed
- Eng C. Int J Oncol. 1998;12:701–710. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous