VIP17/MAL, a lipid raft-associated protein, is involved in apical transport in MDCK cells - PubMed (original) (raw)
VIP17/MAL, a lipid raft-associated protein, is involved in apical transport in MDCK cells
K H Cheong et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Apical proteins are sorted and delivered from the trans-Golgi network to the plasma membrane by a mechanism involving sphingolipid-cholesterol rafts. In this paper, we report the effects of changing the levels of VIP17/MAL, a tetraspan membrane protein localized to post-Golgi transport containers and the apical cell surface in MDCK cells. Overexpression of VIP17/MAL disturbed the morphology of the MDCK cell layers by increasing apical delivery and seemingly expanding the apical cell surface domains. On the other hand, expression of antisense RNA directed against VIP17/MAL caused accumulation in the Golgi and/or impaired apical transport of different apical protein markers, i.e., influenza virus hemagglutinin, the secretory protein clusterin (gp80), the transmembrane protein gp114, and a glycosylphosphatidylinositol-anchored protein. However, antisense RNA expression did not affect the distribution of E-cadherin to the basolateral surface. Because VIP17/MAL associates with sphingolipid-cholesterol rafts, these data provide functional evidence that this protein is involved in apical transport and might be a component of the machinery clustering lipid rafts with apical cargo to form apical transport carriers.
Figures
Figure 1
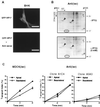
Increased apical delivery of influenza virus HA after induced expression of epitope-tagged VIP17 in MDCK(lac) cells. (A) Comparison of influenza virus HA transport in MDCK strain II and Lac repressor producing MDCK(lac) cells. The cells were infected with influenza virus and incubated for 2 hr to produce viral proteins. Infected cells were labeled for 8 min with [35S]methionine at 37°C and incubated at 19.5°C for 75 min. After release from the 19.5°C block to allow TGN to plasma membrane transport of HA by incubation for 20 min at 37°C, trypsin was added to cleave cell surface HA to HA1 and HA2 on the apical (Ap) or on the basolateral (Bl) surface. In MDCK II cells, 73.5% of HA was delivered to the apical cell surface, whereas only 55.3% of HA was targeted to the apical membrane in MDCK(lac) cells. Molecular weights (kDa) are marked on the left. (B) Expression of epitope-tagged VIP17 under the control of the lac promoter in the VIP17(lac) clone 48, derived from MDCK(lac) cells after transfection of pOPRSVI-VIP17HA. Epitope-tagged VIP17 was detected by mAb 12CA5 as a 19-kDa polypeptide, after induction with 5 mM IPTG for the indicated times. (C) Kinetics of HA transport in MDCK(lac) cells after induced expression of epitope-tagged VIP17 with 5 mM IPTG for 3 days. Influenza-virus infected cells were pulse-labeled with [35S]methionine for 8 min and then kept at 19.5°C for 75 min. After release from the 19.5°C block by increasing the temperature to 37°C for the indicated times, trypsin was added to cleave cell surface HA to HA1 and HA2 on the apical (Ap) or on the basolateral (Bl) surface. Upper images show the SDS/PAGE gels and the Lower images show quantitation of the gels to depict surface delivery of HA to the apical and the basolateral sides. VIP17(lac) clone 48 showed increased apical transport and decreased basolateral mistargeting of HA, as compared with control MDCK(lac) cells.
Figure 2
Decreased apical transport of influenza virus HA after induced expression of antisense RNA against VIP17 in MDCK(lac) cells. (A) Immunofluorescence images of BHK cells that had been transfected with a vector that expressed GFP-tagged VIP17 alone (pOPRSVI-EGFP-VIP17) (Upper) and cotransfected with the vectors that express GFP-VIP17 and antisense RNA against VIP17 (pOPRSVI-EGFP-VIP17 + pOPRSVI-@-VIP17) (Lower). Twenty-four hours after transfection, the cells were fixed and observed in the fluorescence microscope. Expression of GFP-VIP17 was specifically inhibited by cotransfection with the vector expressing antisense RNA. (B) Two-dimensional gel electrophoresis of CHAPS-insoluble proteins from MDCK(lac) cells with (+IPTG) or without (−IPTG) induction of antisense RNA against VIP17. Anti(lac) clones were derived from MDCK(lac) cells after transfection with pOPRSVI-@-VIP17 and selection with G418. After Anti(lac) clone A3A5 was grown with (+IPTG) or without (−IPTG) induction for 3 days, the cells were lysed and processed for two-dimensional gel electrophoresis. VIP17 spot is indicated by a circle and is clearly reduced in antisense-expressing cells as compared with noninduced cells. α- and β-caveolin-1 are marked by arrowheads. (C) Kinetics of HA transport in MDCK(lac) clones, Anti(lac) A1C4 and A5A3 after induction of antisense RNA against VIP17 for 3 days. Both Anti(lac) clones showed reduced apical transport of HA, compared with the control MDCK(lac) cells. [Bar = 10 μm (A).]
Figure 3
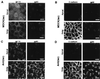
Intracellular localization of endogenous apical and basolateral proteins in MDCK(lac) cells, expressing antisense RNA against VIP17. MDCK(lac) (A and B) and MDCK(lac), expressing antisense RNA against VIP17 [Anti(lac) clone A5A3] (C and D) were grown on coverslips, fixed, and processed for immunofluorescence microscopy and observed under the Zeiss LSM510 confocal microscope. (A and C) Double-immunofluorescence image using antibodies against the apical membrane protein gp114 and the apically secreted protein gp80 in control MDCK(lac) cells and in the Anti(lac) clone A5A3 3 days after induction of antisense expression with IPTG. (B and D) Double-immunofluorescence image using antibodies against basolateral E-cadherin and apically secreted gp80. Upper images show apical surface, and the Golgi region (1 μm below the apical membrane) is visualized in the Lower images in each set. Only apical protein delivery was affected by expressing antisense RNA against VIP17 in MDCK(lac) cells. (Bar = 20 μm.)
Figure 4
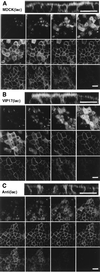
Localization of a fluorescent GPI -anchored protein in MDCK (lac) cells. Filter-grown MDCK(lac) cells (control cell line, VIP17(lac) clone 48 and Anti(lac) clone A5A3) were induced with 5 mM IPTG for 3 days and then infected with recombinant adenovirus for 1 hr to express YFP-GL-GPI and incubated overnight. Cells were fixed with 4% paraformaldehyde and observed under a Zeiss LSM510 microscope. Twelve optical sections (0.5 μm) along the z axis from the apical cell surface to the base of the cell layer are shown, and the Top image is the x_–_z view of the same cells. (A) MDCK(lac) cells infected with recombinant adenoviruses to express YFP-GL-GPI. YFP-GL-GPI localized to the apical and basolateral plasma membrane. (B) VIP17(lac) clone 48, induced for 3 days to express epitope-tagged VIP17, was infected with recombinant adenovirus to express YFP-GL-GPI. YFP-GL-GPI shows stronger signal on the apical membrane when compared with control MDCK(lac) cells. (C) MDCK(lac) cells [Anti(lac) clone A5A3], induced to express antisense RNA against VIP17 for 3 days, were infected with recombinant adenovirus to express YFP-GL-GPI. YFP-GL-GPI was mistargeted to the basolateral membrane. (Bar = 20 μm.)
Figure 5
Localization of apical gp114 and basolateral E-cadherin after adenoviral expression of VIP17 or antisense RNA against VIP17 in MDCK II cells. MDCK II cells were grown on filters and infected with recombinant adenoviruses to express VIP17 or antisense RNA against VIP17 for 1 hr and incubated for 3 days. Cells were fixed and processed by using antibodies against gp114 (Left) and E-cadherin (Right) for immunofluorescence microscopy as in Materials and Methods, and then observed under a Zeiss LSM510 microscope. In each set, three optical x_–_y sections from the apical cell surface to the basal (Upper) and the x_–_z sections (Lower) are shown. (A) In control MDCK II cells, gp114 was localized to the apical and E-cadherin to basolateral plasma membrane. (B) In cells overexpressing VIP17, the apical surface was enlarged, but the targeting of gp114 and E-cadherin was not changed. (C) In cells expressing antisense RNA against VIP17, gp114 was targeted to the basolateral membrane, whereas the localization of basolateral E-cadherin was unaffected. In some cells of the layer, the lateral cell membranes had changed their appearance. (Bar = 20 μm.)
Similar articles
- MAL mediates apical transport of secretory proteins in polarized epithelial Madin-Darby canine kidney cells.
Martín-Belmonte F, Arvan P, Alonso MA. Martín-Belmonte F, et al. J Biol Chem. 2001 Dec 28;276(52):49337-42. doi: 10.1074/jbc.M106882200. Epub 2001 Oct 22. J Biol Chem. 2001. PMID: 11673461 - The MAL proteolipid is necessary for the overall apical delivery of membrane proteins in the polarized epithelial Madin-Darby canine kidney and fischer rat thyroid cell lines.
Martín-Belmonte F, Puertollano R, Millán J, Alonso MA. Martín-Belmonte F, et al. Mol Biol Cell. 2000 Jun;11(6):2033-45. doi: 10.1091/mbc.11.6.2033. Mol Biol Cell. 2000. PMID: 10848627 Free PMC article. - The MAL proteolipid is necessary for normal apical transport and accurate sorting of the influenza virus hemagglutinin in Madin-Darby canine kidney cells.
Puertollano R, Martín-Belmonte F, Millán J, de Marco MC, Albar JP, Kremer L, Alonso MA. Puertollano R, et al. J Cell Biol. 1999 Apr 5;145(1):141-51. doi: 10.1083/jcb.145.1.141. J Cell Biol. 1999. PMID: 10189374 Free PMC article. - MAL, a proteolipid in glycosphingolipid enriched domains: functional implications in myelin and beyond.
Frank M. Frank M. Prog Neurobiol. 2000 Apr;60(6):531-44. doi: 10.1016/s0301-0082(99)00039-8. Prog Neurobiol. 2000. PMID: 10739088 Review. - Expression of MAL and MAL2, two elements of the protein machinery for raft-mediated transport, in normal and neoplastic human tissue.
Marazuela M, Alonso MA. Marazuela M, et al. Histol Histopathol. 2004 Jul;19(3):925-33. doi: 10.14670/HH-19.925. Histol Histopathol. 2004. PMID: 15168355 Review.
Cited by
- The kinesin KIF16B mediates apical transcytosis of transferrin receptor in AP-1B-deficient epithelia.
Perez Bay AE, Schreiner R, Mazzoni F, Carvajal-Gonzalez JM, Gravotta D, Perret E, Lehmann Mantaras G, Zhu YS, Rodriguez-Boulan EJ. Perez Bay AE, et al. EMBO J. 2013 Jul 31;32(15):2125-39. doi: 10.1038/emboj.2013.130. Epub 2013 Jun 7. EMBO J. 2013. PMID: 23749212 Free PMC article. - Function of membrane rafts in viral lifecycles and host cellular response.
Takahashi T, Suzuki T. Takahashi T, et al. Biochem Res Int. 2011;2011:245090. doi: 10.1155/2011/245090. Epub 2011 Dec 7. Biochem Res Int. 2011. PMID: 22191032 Free PMC article. - The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease.
Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, Flask CA, Novick AC, Goldfarb DA, Kramer-Zucker A, Walz G, Piontek KB, Germino GG, Weimbs T. Shillingford JM, et al. Proc Natl Acad Sci U S A. 2006 Apr 4;103(14):5466-71. doi: 10.1073/pnas.0509694103. Epub 2006 Mar 27. Proc Natl Acad Sci U S A. 2006. PMID: 16567633 Free PMC article. - MAL2, a novel raft protein of the MAL family, is an essential component of the machinery for transcytosis in hepatoma HepG2 cells.
de Marco MC, Martín-Belmonte F, Kremer L, Albar JP, Correas I, Vaerman JP, Marazuela M, Byrne JA, Alonso MA. de Marco MC, et al. J Cell Biol. 2002 Oct 14;159(1):37-44. doi: 10.1083/jcb.200206033. Epub 2002 Oct 7. J Cell Biol. 2002. PMID: 12370246 Free PMC article. - Yeast Vps55p, a functional homolog of human obesity receptor gene-related protein, is involved in late endosome to vacuole trafficking.
Belgareh-Touzé N, Avaro S, Rouillé Y, Hoflack B, Haguenauer-Tsapis R. Belgareh-Touzé N, et al. Mol Biol Cell. 2002 May;13(5):1694-708. doi: 10.1091/mbc.01-12-0597. Mol Biol Cell. 2002. PMID: 12006663 Free PMC article.
References
- Rodriguez-Boulan E, Powell S K. Annu Rev Cell Biol. 1992;8:395–427. - PubMed
- Simons K, Ikonen E. Nature (London) 1997;387:569–572. - PubMed
- Matter K, Mellman I. Curr Opin Cell Biol. 1994;6:545–554. - PubMed
- Ikonen E, Simons K. Semin Cell Dev Biol. 1998;9:503–509. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials