p27 and Rb are on overlapping pathways suppressing tumorigenesis in mice - PubMed (original) (raw)
p27 and Rb are on overlapping pathways suppressing tumorigenesis in mice
M S Park et al. Proc Natl Acad Sci U S A. 1999.
Abstract
The commitment of cells to replicate and divide correlates with the activation of cyclin-dependent kinases and the inactivation of Rb, the product of the retinoblastoma tumor suppressor gene. Rb is a target of the cyclin-dependent kinases and, when phosphorylated, is inactivated. Biochemical studies exploring the nature of the relationship between cyclin-dependent kinase inhibitors and Rb have supported the hypothesis that these proteins are on a linear pathway regulating commitment. We have been able to study this relationship by genetic means by examining the phenotype of Rb+/-p27-/- mice. Tumors arise from the intermediate lobe cells of the pituitary gland in p27-/- mice, as well as in Rb+/- mice after loss of the remaining wild-type allele of Rb. Using these mouse models, we examined the genetic interaction between Rb and p27. We found that the development of pituitary tumors in Rb+/- mice correlated with a reduction in p27 mRNA and protein expression. To determine whether the loss of p27 was an indirect consequence of tumor formation or a contributing factor to the development of this tumor, we analyzed the phenotype of Rb+/-p27-/- mice. We found that these mice developed pituitary adenocarcinoma with loss of the remaining wild-type allele of Rb and a high-grade thyroid C cell carcinoma that was more aggressive than the disease in either Rb+/- or p27-/- mice. Importantly, we detected both pituitary and thyroid tumors earlier in the Rb+/-p27-/- mice. We therefore propose that Rb and p27 cooperate to suppress tumor development by integrating different regulatory signals.
Figures
Figure 1
p27 protein expression is reduced in pituitary tumors arising in Rb heterozygous mice. The specificity of the p27 antibody was determined by comparing the reactivity in wild-type pituitary cells (a) and wild-type adjacent brain tissue (b). Pituitary reactivity could be blocked by inclusion of excess antigen (c) or exclusion of primary antibody (d). Note the staining of p27 is greater in the pituitary cells than in the adjacent brain cells. A reciprocal pattern of accumulation is observed when comparing the pituitary tumor (e) and normal brain tissue (f) obtained from Rb+/− mice. (Magnification: ×40.)
Figure 2
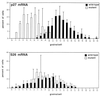
p27 mRNA expression is reduced in pituitary tumors arising in Rb heterozygous mice. The percentage of cells with a particular number of grains is plotted on the ordinate. The data from a representative experiment are shown. (Top) The distribution of p27 mRNA. (Bottom) The distribution of S26 mRNA (a nonspecific control). Wild-type tissue is represented by black bars, and tumor tissue is represented by the white bars.
Figure 3
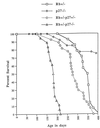
Survival of mice with mutations in Rb and p27. The graph summarizes the viability of mice for each genotype (n = 30, p27_−/−; n = 10, Rb+/−; n = 20, Rb+/−_p27+/−; n = 30, Rb+/−_p27_−/−). The mean age of survival is given in Results and Discussion.
Figure 4
Pituitary tumorigenesis. (a–c) Representative sections through Rb+/−, _p27_−/−, and Rb+/−_p27_−/− tissues and tumors are shown at ×40 magnification. (d–f) Higher magnification (×100) of the different cell types observed in the Rb+/−_p27_−/− tumor. (g) Small-cell phenotype correlates with the largest tumor volumes. The tumor volume is plotted on the ordinate and is shown as a function of the absence (left bar) or presence (right bar) of the small-cell-morphology phenotype. The bars represent the mean, and the SD is included for the samples, each indicated by a character. (h) Rb genotype in the pituitary tumors. The loss of Rb was determined by Southern blotting DNA prepared from two tissues of either Rb+/− or Rb+/−_p27_−/− animals. As a control for the wild-type (wt) allele, tail DNA obtained from the animal was processed similarly. The source of tissue is indicated above each lane. The genotype of the animal is below each panel, and the migration of the Rb allele is on the left. We used only tail DNA from an Rb+/− animal.
Figure 5
Thyroid tumorigenesis. (A) Morphologic and immunohistochemical features of the thyroid. Hematoxylin and eosin staining (a) shows the presence of large follicles surrounded by C cells (chromogranin-positive cells in c) and thyroglobulin-producing cells (b). (Magnification: ×40.) (B) Representative sections through Rb+/− (a and b), _p27_−/− (c and d), and Rb+/−_p27_−/− (e and f). (a, c, and e, magnification: ×40. b, d, and f, magnification: ×100.) a, c, and e represent the two lobes of the thyroid.
References
- Dyson N. Genes Dev. 1998;12:2245–2262. - PubMed
- Nevins J R. Cell Growth Differ. 1998;9:585–593. - PubMed
- Sheaff R J, Roberts J M. In: Cell Cycle Control. Pagano M, editor. New York: Springer; 1998. pp. pp.1–34.
- Zarkowska T, Mittnacht S. J Biol Chem. 1997;272:12738–12746. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases