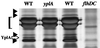A new pathway for the secretion of virulence factors by bacteria: the flagellar export apparatus functions as a protein-secretion system - PubMed (original) (raw)
A new pathway for the secretion of virulence factors by bacteria: the flagellar export apparatus functions as a protein-secretion system
G M Young et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Biogenesis of the flagellum, a motive organelle of many bacterial species, is best understood for members of the Enterobacteriaceae. The flagellum is a heterooligomeric structure that protrudes from the surface of the cell. Its assembly initially involves the synthesis of a dedicated protein export apparatus that subsequently transports other flagellar proteins by a type III mechanism from the cytoplasm to the outer surface of the cell, where oligomerization occurs. In this study, the flagellum export apparatus was shown to function also as a secretion system for the transport of several extracellular proteins in the pathogenic bacterium Yersinia enterocolitica. One of the proteins exported by the flagellar secretion system was the virulence-associated phospholipase, YplA. These results suggest type III protein secretion by the flagellar system may be a general mechanism for the transport of proteins that influence bacterial-host interactions.
Figures
Figure 1
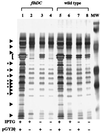
Extracellular protein secretion by Y. enterocolitica requires the flagellar export apparatus. Extracellular proteins were concentrated from culture supernatants, separated by SDS/PAGE in 12.5% gels, and stained with silver. Lanes: 1, GY460/pGY15; 2, YMS12/pMS_flh_; 3, YMS13/pMS_flh_; 4, GY460 (flhDC); 5, YMS12 (flhA); 6, YMS13 (flhB); 7, JO1v (flgM); 8, JO1v/pSWIM1; 9, VK1/pJB222; 10, VK1 (fliA); and 11, JB580v (wild type). Each lane contained the equivalent of 3 ml supernatant from a culture at an OD600 of 1.0. Molecular mass standards (MW) from top to bottom are 81, 46.9, 34.1, 28.5, and 20 kDa. The location of flagellin is indicated by the bracket at the left. The locations of Fops are indicated by arrows (regions with a single species) and arrows with asterisks to denote regions where multiple species comigrate.
Figure 2
Fop production is affected by expression of the flagellar regulon. Extracellular proteins were examined from concentrated culture supernatants of strains grown under conditions that modulate the expression of motility. Expression of motility was modulated by expressing flhDC from a p_tac_ promoter. Transcription from the p_tac_ promoter was positively controlled by the inclusion of IPTG in the growth medium. Proteins were separated by SDS/PAGE and stained as described in Fig. 1. Lanes: 1, GY460/pGY20 grown in T medium + 50 μM IPTG; 2, GY460/pVLT33 grown in T medium + 50 μM IPTG; 3, GY460/pGY20 grown in T medium; 4, GY460/pVLT33 grown in T medium; 5, JB580v/pGY20 grown in T medium + 50 μM IPTG; 6, JB580v/pVLT33 grown in T medium + 50 μM IPTG; 7, JB580v/pGY20 grown in T medium; and 8, JB580v/pVLT33 grown in T medium. Each lane contains the equivalent of 1.5 ml supernatant from a culture at an OD600 of 1.0. The locations of Fops are indicated by arrows (regions with a single species) and arrows with asterisks to denote regions where multiple species comigrate.
Figure 3
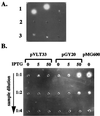
YplA is an extracellular protein whose production requires expression of the flagellar regulon. (A) Concentrated whole-cell lysates and culture supernatants of strain JB580v grown in LB or T medium were examined for phospholipase activity by a radial diffusion assay. A positive reaction resulted in the formation of a precipitate emanating from the sample well in gels containing the artificial substrate Tween 80. Each well contained an equivalent of cells or supernatants from 1.5 ml culture at an OD600 of 3.0. Row 1, culture supernatant after growth in T medium (Left), culture supernatant after growth in T medium and preheated to 90°C for 10 min (Center), and cell lysate after growth in T medium (Right). Row 2, culture supernatant after growth in LB (Left), culture supernatant after growth in LB and preheated to 90°C for 10 min (Center), and cell lysate after growth in T medium and preheated to 90°C for 10 min (Right). Row 3, cell lysates after growth in LB (Left), cell lysate after growth in LB and preheated to 90°C for 10 min (Center), and buffer control (Right). (B) Phospholipase activity of secreted YplA was measured for strain GY460 containing plasmid pVLT33 (vector control), pGY20 (p_tac-flhDC_ from Y. enterocolitica), and pMG600 (p_tac-flhDC_ from S. liquefaciens). Conditions of the assay were as described in A. Each well contained concentrated culture supernatant for each strain grown in T medium, with the inducer IPTG at a concentration of 0, 5, or 50 μM (indicated at the top). From top to bottom each well was loaded with an increased dilution of each sample.
Figure 4
Identification of YplA as a 35.5-kDa secreted protein. Extracellular proteins were concentrated from culture supernatants of strains YEDS10/pGY20, GY460/pGY20, and GY460/pVLT33 grown in T medium with 50 μM IPTG to induced transcription from the p_tac_ promoter. Proteins were separated on 12.5% SDS-polyacrylamide gel and stained with silver. Lanes: 1, GY460/pGY20; 2, YEDS10/pGY20; 3, GY460/pGY20; and 4, GY460/pVLT33. Each lane contains the equivalent of 1.5 ml culture at an OD600 of 1.0. The position of YplA is marked with an open arrow, and other Fops are marked with solid arrowheads. The location of flagellin is indicated with a bracket.
Figure 5
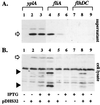
Detection of YplA in culture supernatants by immunoblot analysis. Concentrated protein from whole-cell lysates or culture supernatants from strains grown in T medium with 0 and 100 μM IPTG were separated on 12.5% SDS-polyacrylamide gel, transferred to nitrocellulose membranes, and probed with anti-YplA antibody. (A) Levels of YplA in culture supernatants. Lanes: 1, YEDS10, 0 μM IPTG; 2, YEDS10/pDHS32, 0 μM IPTG; 3, YEDS10/pDHS32, 100 μM IPTG; 4, VK1/pDHS32, 100 μM IPTG; 5, VK1/pDHS32, 0 μM IPTG; 6, VK1/pVLT33, 100 μM IPTG; 7, GY460/pVLT33, 100 μM IPTG; 8, GY460/pDHS32, 100 μM IPTG; and 9, GY460, 0 μM IPTG. (B) Levels of YplA detected in whole cells. Lanes are the same as those listed in A. Full-length YplA is indicated by the open arrow. YplA degradation products detected in whole cells are indicated by the solid arrowheads.
Similar articles
- An amino-terminal secretion signal is required for YplA export by the Ysa, Ysc, and flagellar type III secretion systems of Yersinia enterocolitica biovar 1B.
Warren SM, Young GM. Warren SM, et al. J Bacteriol. 2005 Sep;187(17):6075-83. doi: 10.1128/JB.187.17.6075-6083.2005. J Bacteriol. 2005. PMID: 16109949 Free PMC article. - The Yersinia enterocolitica phospholipase gene yplA is part of the flagellar regulon.
Schmiel DH, Young GM, Miller VL. Schmiel DH, et al. J Bacteriol. 2000 Apr;182(8):2314-20. doi: 10.1128/JB.182.8.2314-2320.2000. J Bacteriol. 2000. PMID: 10735878 Free PMC article. - YplA is exported by the Ysc, Ysa, and flagellar type III secretion systems of Yersinia enterocolitica.
Young BM, Young GM. Young BM, et al. J Bacteriol. 2002 Mar;184(5):1324-34. doi: 10.1128/JB.184.5.1324-1334.2002. J Bacteriol. 2002. PMID: 11844761 Free PMC article. - A rationale for repression and/or loss of motility by pathogenic Yersinia in the mammalian host.
Minnich SA, Rohde HN. Minnich SA, et al. Adv Exp Med Biol. 2007;603:298-310. doi: 10.1007/978-0-387-72124-8_27. Adv Exp Med Biol. 2007. PMID: 17966426 Review. - How bacteria assemble flagella.
Macnab RM. Macnab RM. Annu Rev Microbiol. 2003;57:77-100. doi: 10.1146/annurev.micro.57.030502.090832. Epub 2003 May 1. Annu Rev Microbiol. 2003. PMID: 12730325 Review.
Cited by
- Giardia duodenalis-induced alterations of commensal bacteria kill Caenorhabditis elegans: a new model to study microbial-microbial interactions in the gut.
Gerbaba TK, Gupta P, Rioux K, Hansen D, Buret AG. Gerbaba TK, et al. Am J Physiol Gastrointest Liver Physiol. 2015 Mar 15;308(6):G550-61. doi: 10.1152/ajpgi.00335.2014. Epub 2015 Jan 8. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 25573177 Free PMC article. - CsrA impacts survival of Yersinia enterocolitica by affecting a myriad of physiological activities.
LeGrand K, Petersen S, Zheng Y, Liu KK, Ozturk G, Chen JY, Young GM. LeGrand K, et al. BMC Microbiol. 2015 Feb 14;15:31. doi: 10.1186/s12866-015-0343-6. BMC Microbiol. 2015. PMID: 25885058 Free PMC article. - Genetic determinants of swimming motility in the squid light-organ symbiont Vibrio fischeri.
Brennan CA, Mandel MJ, Gyllborg MC, Thomasgard KA, Ruby EG. Brennan CA, et al. Microbiologyopen. 2013 Aug;2(4):576-94. doi: 10.1002/mbo3.96. Epub 2013 Jun 12. Microbiologyopen. 2013. PMID: 23907990 Free PMC article. - Secretion of Salmonella Pathogenicity Island 1-Encoded Type III Secretion System Effectors by Outer Membrane Vesicles in Salmonella enterica Serovar Typhimurium.
Kim SI, Kim S, Kim E, Hwang SY, Yoon H. Kim SI, et al. Front Microbiol. 2018 Nov 23;9:2810. doi: 10.3389/fmicb.2018.02810. eCollection 2018. Front Microbiol. 2018. PMID: 30532744 Free PMC article. - Pathogenicity of Serratia marcescens Strains in Honey Bees.
Raymann K, Coon KL, Shaffer Z, Salisbury S, Moran NA. Raymann K, et al. mBio. 2018 Oct 9;9(5):e01649-18. doi: 10.1128/mBio.01649-18. mBio. 2018. PMID: 30301854 Free PMC article.
References
- Wandersman C. In: Escherichia coli and Salmonella: Cellular and Molecular Biology. Neidhardt F C, editor. Vol. 1. Washington, DC: Am. Soc. Microbiol.; 1996. pp. 955–966.
- Galan J E, Sansonetti P J. In: Escherichia coli and Salmonella: Cellular and Molecular Biology. Neidhardt F C, editor. Vol. 2. Washington, DC: Am. Soc. Microbiol.; 1996. pp. 2757–2773.
- MacNab R M. In: Escherichia coli and Salmonella: Cellular and Molecular Biology. Neidhardt F C, editor. Vol. 1. Washington, DC: Am. Soc. Microbiol.; 1996. pp. 123–145.
- Aizawa S. Mol Microbiol. 1996;19:1–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources