Signaling by proinflammatory cytokines: oligomerization of TRAF2 and TRAF6 is sufficient for JNK and IKK activation and target gene induction via an amino-terminal effector domain - PubMed (original) (raw)
Signaling by proinflammatory cytokines: oligomerization of TRAF2 and TRAF6 is sufficient for JNK and IKK activation and target gene induction via an amino-terminal effector domain
V Baud et al. Genes Dev. 1999.
Abstract
Interleukin-1 (IL-1) and tumor necrosis factor (TNF-alpha) stimulate transcription factors AP-1 and NF-kappaB through activation of the MAP kinases JNK and p38 and the IkappaB kinase (IKK), respectively. The TNF-alpha and IL-1 signals are transduced through TRAF2 and TRAF6, respectively. Overexpressed TRAF2 or TRAF6 activate JNK, p38, or IKK in the absence of extracellular stimulation. By replacing the carboxy-terminal TRAF domain of TRAF2 and TRAF6 with repeats of the immunophilin FKBP12, we demonstrate that their effector domains are composed of their amino-terminal Zn and RING fingers. Oligomerization of the TRAF2 effector domain results in specific binding to MEKK1, a protein kinase capable of JNK, p38, and IKK activation, and induction of TNF-alpha and IL-1 responsive genes. TNF-alpha also enhances the binding of native TRAF2 to MEKK1 and stimulates the kinase activity of the latter. Thus, TNF-alpha and IL-1 signaling is based on oligomerization of TRAF2 and TRAF6 leading to activation of effector kinases.
Figures
Figure 1
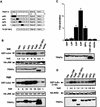
TRAF6 mediates JNK and p38 activation by IL-1. (A) HEK293 cells were cotransfected with HA-JNK1 (0.5 μg/plate) along with either an empty expression vector (Vec), IRAK or IRAK(Δ218–507) (100 ng/plate each), or TRAF6 or TRAF6(289–522) (1 μg/plate each) expression vectors. Total DNA was kept constant (1.5 μg/plate) using empty expression vector. After 24 hr the transfected cells were treated with IL-1 (10 ng/ml) for 30 min or left untreated. Cells were collected, lysed, and HA–JNK1 activity was determined by immunocomplex kinase assay with GST-cJun(1–79) as a substrate. Fold-increase in HA–JNK1 activity above the basal level in cells cotransfected with empty expression vector was determined by PhosphorImaging and normalized to the level of HA–JNK1 expression, determined by immunoblotting. (B) HEK293 cells were transfected as described above except that an HA–p38α vector (0.5 μg/plate) was used instead of the HA–JNK1 vector. HA–p38α activity was determined, as above, by immunocomplex kinase assay with myelin basic protein (MBP) as a substrate. (C) HEK293 cells were cotransfected with HA–JNK1 (0.5 μg/plate), IRAK or IRAK(Δ218–507) (100 ng/plate each), TRAF6 or TRAF6(289–522) (1 μg/plate each) expression vectors as indicated. After 24 hr some cultures were treated with IL-1 for 30 min and the rest left untreated. HA–JNK1 activity and expression were determined as described above. Expression of Flag–TRAF6, Flag–TRAF6(289–522), or IRAK was determined by immunoblotting.
Figure 2
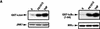
TRAF–FKBP12 chimeras activate JNK and NF-κB in response to dimerizer-induced oligomerization. (A) A diagram illustrating the domain structure of the two TRAF–FKBP12 fusion proteins. (B) HEK293 cells were transfected with HA–JNK1 (0.5 μg/plate) along with either empty expression vector or expression vectors for Flag–TRAF2 or Flag–TRAF2(1–303)–FKBP12 (1 μg/plate each). Some of the transfected cells were treated with either TNF-α (15 ng/ml) for 10 min, or FK506 (0.5 μ
m
) or FK1012 (0.5 μ
m
) for 4 hr, as indicated. JNK activity was determined as described above. Expression of HA–JNK1, Flag–TRAF2, or Flag–TRAF2(1–303)–FKBP12 were examined by immunoblotting. Similar results were obtained using HeLa cells as recipients (data not shown). (C) Similar experiments to those described in B were performed using Flag–TRAF6 and Flag–TRAF6(1–274)–FKBP12 expression vectors. Where indicated, transfected cells were treated with IL-1 (4 ng/ml) for 10 min (D, E) NF-κB activation by TRAF2(1–303)–FKBP12 (D) or TRAF6(1–274)–FKBP12 (E) was measured by transfecting HEK293 cells with a 2×NF–κB–LUC reporter (0.5 μg/plate) and the indicated TRAF and TRAF–FKBP12 expression vectors (1 μg/plate). pRSV–lacZ (0.1 μg/plate) was included to normalize transfection efficiency. Cells were collected 6 hr after the indicated treatments (as in B), which were initiated 24 hr after transfection, and luciferase activity was determined. (F) HeLa cells were transfected with Flag–IKKα (0.5 μg) and the indicated TRAF2–FKBP12 vectors. Where indicated, cells were treated with TNF-α for 5 min or FK1012 for 4 hr before collection and lysis. Immunocomplex kinase assay was performed with GST–IκBα(1–54) as a substrate.
Figure 3
Activation of JNK and NF-κB by TRAF2–FKBP12 is insensitive to TANK/I–TRAF. (A) HEK293 cells were cotransfected with HA–JNK1 (0.5 μg/plate) along with an empty expression vector, Flag–TRAF2 or Flag–TRAF2(1–303)–FKBP12 (500 ng/plate each) and I–TRAF (150 ng/plate) expression vectors as indicated. Cells were treated with either TNF-α for 15 min, or FK506 or FK1012 for 4 hr, as indicated, before being collected to measure HA–JNK1 activity and expression. (B) HEK293 cells were transfected with 2×NF–κB–LUC and RSV–lacZ reporters along with the indicated expression vectors and treated as in A. Luciferase activity was determined as described above.
Figure 3
Activation of JNK and NF-κB by TRAF2–FKBP12 is insensitive to TANK/I–TRAF. (A) HEK293 cells were cotransfected with HA–JNK1 (0.5 μg/plate) along with an empty expression vector, Flag–TRAF2 or Flag–TRAF2(1–303)–FKBP12 (500 ng/plate each) and I–TRAF (150 ng/plate) expression vectors as indicated. Cells were treated with either TNF-α for 15 min, or FK506 or FK1012 for 4 hr, as indicated, before being collected to measure HA–JNK1 activity and expression. (B) HEK293 cells were transfected with 2×NF–κB–LUC and RSV–lacZ reporters along with the indicated expression vectors and treated as in A. Luciferase activity was determined as described above.
Figure 4
Mutations within the amino-terminal effector domain of TRAF2 differentially affect JNK and IKK activation. (A) Schematic representation of TRAF2 mutants. The numbers below the diagram refer to amino acids positions. F1–5 denote the five Zn fingers. The domains in which pairs of cysteine residues were replaced with serines are indicated by black boxes (mR1: S34/37; mF1: S107/112; mF2: S136/139; and mF3: S163/166). (B) JNK activation. HEK293 cells were cotransfected with HA–JNK1 and either an empty vector or expression vectors encoding wild-type or mutant versions of TRAF2, used at either low (0.2 μg/plate; top two panels) or high (1 μg/plate; bottom two panels) input levels. After 24 hr cells were collected and HA–JNK1 activity and expression were determined. TRAF expression was also determined. (C) NF-κB activation. HEK293 cells were cotransfected with 2×NF–κB–LUC and pRSV–lacZ reporters and either an empty vector or expression vectors for wild-type and mutant TRAF2 proteins as indicated (1 μg/plate). Cells were collected, and luciferase activity and TRAF expression were determined. (D) IKK activation. HEK293 cells were cotransfected with HA–IKKβ (0.25 μg/plate) and either an empty vector or expression vectors for wild-type and mutant TRAF2 proteins (1 μg/plate). Some transfectants were treated with TNF-α (15 ng/ml) for 10 min. Immunocomplex kinase assay was performed with GST–IκBα(1–54) as a substrate. HA–IKKβ and TRAF expression were determined by immunoblotting.
Figure 5
Treatment of HeLa cells stably expressing TRAF2–FKBP12 with FK1012 activates the inflammation-induced gene expression program. (A) Treatment of HeLa cells that stably express TRAF2(1–303)–FKBP12 with FK1012 activates JNK and IKK. Endogenous JNK1 (left) or IKKα (right) was immunoprecipitated from untreated or cells treated with either TNF-α (15 ng/ml for 10 min) or FK1012 (0.5 μ
m
for 4 hr). JNK and IKK activity were determined by immunocomplex kinase assays with GST–cJun(1–79) and GST–IκBα(1–54) as substrates, respectively. Immunoprecipitated JNK1 and IKKα proteins were detected by immunoblotting. (B) Oligomerization of TRAF2(1–303)–FKBP12 induces the same gene expression program as TNF-α or IL-1. Membranes containing an array of 597 spotted cDNA fragments derived from different human genes were hybridized to 32P-labeled cDNA probes derived from poly(A)+ RNA samples prepared from either parental HeLa cells that were either untreated or treated with either TNF-α (solid bars; 15 ng/ml) or IL-1 (hatched bars; 4 ng/ml) for 7 hr, or from HeLa cells stably expressing TRAF2(1–303)–FKBP12 that were either untreated or treated with FK1012 (open bars; 0.5 μ
m
for 4 hr). The fold-increase in gene expression above the level in untreated parental or transfected cells was determined by PhosphorImaging and a series of Excel-based macros as described in Materials and Methods. Data are averages of two completely independent experiments. Only 4 of the 590 genes whose expression was not induced by TNF-α, IL-1, or FK1012 are included. Treatment of parental HeLa cells with FK1012 did not result in gene induction (data not shown).
Figure 5
Treatment of HeLa cells stably expressing TRAF2–FKBP12 with FK1012 activates the inflammation-induced gene expression program. (A) Treatment of HeLa cells that stably express TRAF2(1–303)–FKBP12 with FK1012 activates JNK and IKK. Endogenous JNK1 (left) or IKKα (right) was immunoprecipitated from untreated or cells treated with either TNF-α (15 ng/ml for 10 min) or FK1012 (0.5 μ
m
for 4 hr). JNK and IKK activity were determined by immunocomplex kinase assays with GST–cJun(1–79) and GST–IκBα(1–54) as substrates, respectively. Immunoprecipitated JNK1 and IKKα proteins were detected by immunoblotting. (B) Oligomerization of TRAF2(1–303)–FKBP12 induces the same gene expression program as TNF-α or IL-1. Membranes containing an array of 597 spotted cDNA fragments derived from different human genes were hybridized to 32P-labeled cDNA probes derived from poly(A)+ RNA samples prepared from either parental HeLa cells that were either untreated or treated with either TNF-α (solid bars; 15 ng/ml) or IL-1 (hatched bars; 4 ng/ml) for 7 hr, or from HeLa cells stably expressing TRAF2(1–303)–FKBP12 that were either untreated or treated with FK1012 (open bars; 0.5 μ
m
for 4 hr). The fold-increase in gene expression above the level in untreated parental or transfected cells was determined by PhosphorImaging and a series of Excel-based macros as described in Materials and Methods. Data are averages of two completely independent experiments. Only 4 of the 590 genes whose expression was not induced by TNF-α, IL-1, or FK1012 are included. Treatment of parental HeLa cells with FK1012 did not result in gene induction (data not shown).
Figure 6
Oligomerization-induced interaction of TRAF2–FKBP12 with MEKK1. HEK293 cells were transfected with 0.4 μg of different expression vectors encoding wild-type or mutant versions of TRAF2–FKBP12 chimeras and different MAPKKKs (MAP3K; MEKK1, MEKK2, ASK1, NIK) or STE20-like kinases (MAP4K; GCK), as indicated. The transfected cultures were left untreated or treated with FK1012 (0.5 μ
m
for 4 hr) before lysis in nondenaturing buffer. After centrifugation the dissolved insoluble (P) and soluble (S) fractions were separated by 7.5% SDS–polyacrylamide gel. MEKK1, ASK, GCK, and NIK were detected by immunoblotting with antibody to their amino-terminal Xpress tag. The 35K and 70K MEKK1ΔN truncation mutants and MEKK2 were detected with anti-MEKK1 and MEKK2, respectively. The different TRAF2–FKBP12 chimeras were detected with an antibody to their amino-terminal Flag tag. Equal loading of the lanes was controlled by probing the same blots with an actin antibody.
Figure 7
TNF-α activates MEKK1 and enhances its binding to TRAF2. (A) TNF-α-dependent association of TRAF2 with MEKK1 and increase of MEKK1 autokinase activity. HEK293 cells were cotransfected with Flag–TRAF2 (50 ng DNA/plate) along with either an empty vector or Xpress–MEKK1 (100 ng DNA/plate). Lysates prepared from untreated or TNF-α-treated cells (10 ng/ml, 4 min) were immunoprecipitated with anti-MEKK1. Coprecipitating Flag–TRAF2 was detected by immunoblot analysis. MEKK1 autophosphorylation was determined by immunocomplex kinase assay without exogenous substrate. Fold increase in TNF-α-induced MEKK1 autophosphorylation was determined by PhosphorImaging. (B) Activation of endogenous MEKK1 by TNF-α. HeLa cells were treated with TNF-α for 30 min, 1 hr, or left untreated. Endogenous MEKK1 was immunoprecipitated from 1 mg of total cell lysates and its autokinase activity and ability to phosphorylate catalytically inactive JNKK1 were determined by immunocomplex kinase assay. (C) Inactive MEKK1 mutant inhibits JNK activation by TRAF2 and TRAF2–FKBP12. HEK293 cells were cotransfected with HA–JNK1 (0.4 μg DNA/plate), along with an empty expression vector, Flag–TRAF2 (50 ng DNA/plate), or Flag–TRAF2–FKBP12 (100 ng DNA/plate) and MEKK1 (K432M) (2 μg DNA/plate) expression vectors as indicated. Cells were left untreated or treated with FK1012 for 4 hr, as indicated, before being collected to measure HA–JNK1 activity and expression.
Figure 7
TNF-α activates MEKK1 and enhances its binding to TRAF2. (A) TNF-α-dependent association of TRAF2 with MEKK1 and increase of MEKK1 autokinase activity. HEK293 cells were cotransfected with Flag–TRAF2 (50 ng DNA/plate) along with either an empty vector or Xpress–MEKK1 (100 ng DNA/plate). Lysates prepared from untreated or TNF-α-treated cells (10 ng/ml, 4 min) were immunoprecipitated with anti-MEKK1. Coprecipitating Flag–TRAF2 was detected by immunoblot analysis. MEKK1 autophosphorylation was determined by immunocomplex kinase assay without exogenous substrate. Fold increase in TNF-α-induced MEKK1 autophosphorylation was determined by PhosphorImaging. (B) Activation of endogenous MEKK1 by TNF-α. HeLa cells were treated with TNF-α for 30 min, 1 hr, or left untreated. Endogenous MEKK1 was immunoprecipitated from 1 mg of total cell lysates and its autokinase activity and ability to phosphorylate catalytically inactive JNKK1 were determined by immunocomplex kinase assay. (C) Inactive MEKK1 mutant inhibits JNK activation by TRAF2 and TRAF2–FKBP12. HEK293 cells were cotransfected with HA–JNK1 (0.4 μg DNA/plate), along with an empty expression vector, Flag–TRAF2 (50 ng DNA/plate), or Flag–TRAF2–FKBP12 (100 ng DNA/plate) and MEKK1 (K432M) (2 μg DNA/plate) expression vectors as indicated. Cells were left untreated or treated with FK1012 for 4 hr, as indicated, before being collected to measure HA–JNK1 activity and expression.
Figure 7
TNF-α activates MEKK1 and enhances its binding to TRAF2. (A) TNF-α-dependent association of TRAF2 with MEKK1 and increase of MEKK1 autokinase activity. HEK293 cells were cotransfected with Flag–TRAF2 (50 ng DNA/plate) along with either an empty vector or Xpress–MEKK1 (100 ng DNA/plate). Lysates prepared from untreated or TNF-α-treated cells (10 ng/ml, 4 min) were immunoprecipitated with anti-MEKK1. Coprecipitating Flag–TRAF2 was detected by immunoblot analysis. MEKK1 autophosphorylation was determined by immunocomplex kinase assay without exogenous substrate. Fold increase in TNF-α-induced MEKK1 autophosphorylation was determined by PhosphorImaging. (B) Activation of endogenous MEKK1 by TNF-α. HeLa cells were treated with TNF-α for 30 min, 1 hr, or left untreated. Endogenous MEKK1 was immunoprecipitated from 1 mg of total cell lysates and its autokinase activity and ability to phosphorylate catalytically inactive JNKK1 were determined by immunocomplex kinase assay. (C) Inactive MEKK1 mutant inhibits JNK activation by TRAF2 and TRAF2–FKBP12. HEK293 cells were cotransfected with HA–JNK1 (0.4 μg DNA/plate), along with an empty expression vector, Flag–TRAF2 (50 ng DNA/plate), or Flag–TRAF2–FKBP12 (100 ng DNA/plate) and MEKK1 (K432M) (2 μg DNA/plate) expression vectors as indicated. Cells were left untreated or treated with FK1012 for 4 hr, as indicated, before being collected to measure HA–JNK1 activity and expression.
Similar articles
- Differential requirements for tumor necrosis factor receptor-associated factor family proteins in CD40-mediated induction of NF-kappaB and Jun N-terminal kinase activation.
Leo E, Welsh K, Matsuzawa S, Zapata JM, Kitada S, Mitchell RS, Ely KR, Reed JC. Leo E, et al. J Biol Chem. 1999 Aug 6;274(32):22414-22. doi: 10.1074/jbc.274.32.22414. J Biol Chem. 1999. PMID: 10428814 - Requirement of tumor necrosis factor receptor-associated factor (TRAF)6 in interleukin 17 signal transduction.
Schwandner R, Yamaguchi K, Cao Z. Schwandner R, et al. J Exp Med. 2000 Apr 3;191(7):1233-40. doi: 10.1084/jem.191.7.1233. J Exp Med. 2000. PMID: 10748240 Free PMC article. - Regulation and function of IKK and IKK-related kinases.
Häcker H, Karin M. Häcker H, et al. Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review.
Cited by
- Regulation of Mycobacterium tuberculosis-dependent HIV-1 transcription reveals a new role for NFAT5 in the toll-like receptor pathway.
Ranjbar S, Jasenosky LD, Chow N, Goldfeld AE. Ranjbar S, et al. PLoS Pathog. 2012;8(4):e1002620. doi: 10.1371/journal.ppat.1002620. Epub 2012 Apr 5. PLoS Pathog. 2012. PMID: 22496647 Free PMC article. - TRAF3 as a powerful and multitalented regulator of lymphocyte functions.
Bishop GA. Bishop GA. J Leukoc Biol. 2016 Nov;100(5):919-926. doi: 10.1189/jlb.2MR0216-063R. Epub 2016 May 6. J Leukoc Biol. 2016. PMID: 27154354 Free PMC article. Review. - MEKK1 plays a critical role in activating the transcription factor C/EBP-beta-dependent gene expression in response to IFN-gamma.
Roy SK, Hu J, Meng Q, Xia Y, Shapiro PS, Reddy SP, Platanias LC, Lindner DJ, Johnson PF, Pritchard C, Pagés G, Pouyssegur J, Kalvakolanu DV. Roy SK, et al. Proc Natl Acad Sci U S A. 2002 Jun 11;99(12):7945-50. doi: 10.1073/pnas.122075799. Epub 2002 Jun 4. Proc Natl Acad Sci U S A. 2002. PMID: 12048245 Free PMC article. - Etk/Bmx as a tumor necrosis factor receptor type 2-specific kinase: role in endothelial cell migration and angiogenesis.
Pan S, An P, Zhang R, He X, Yin G, Min W. Pan S, et al. Mol Cell Biol. 2002 Nov;22(21):7512-23. doi: 10.1128/MCB.22.21.7512-7523.2002. Mol Cell Biol. 2002. PMID: 12370298 Free PMC article. - The interferon signaling network and transcription factor C/EBP-beta.
Li H, Gade P, Xiao W, Kalvakolanu DV. Li H, et al. Cell Mol Immunol. 2007 Dec;4(6):407-18. Cell Mol Immunol. 2007. PMID: 18163952 Free PMC article. Review.
References
- Arch RH, Gedrich RW, Thompson CB. Tumor necrosis factor receptor-associated factors (TRAFs)—A family of adapter proteins that regulates life and death. Genes & Dev. 1998;12:2821–2830. - PubMed
- Baeuerle PA, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. - PubMed
- Barnes PJ, Karin M. Nuclear factor-κB—A pivotal transcription factor in chronic inflammatory diseases. New Engl J Med. 1997;336:1066–1071. - PubMed
- Beg AA, Baldwin AS., Jr The IκB proteins: Multifunctional regulators of Rel/NF-κB transcription factors. Genes & Dev. 1993;7:2064–2070. - PubMed
- Blank JL, Gerwins P, Elliott EM, Sather S, Johnson GL. Molecular cloning of mitogen-activated protein/ERK kinase kinases (MEKK) 2 and 3. J Biol Chem. 1996;271:5361–5368. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 AI043477/AI/NIAID NIH HHS/United States
- AI43477-01/AI/NIAID NIH HHS/United States
- R37 ES004151/ES/NIEHS NIH HHS/United States
- DK38527-11/DK/NIDDK NIH HHS/United States
- ES04151-13/ES/NIEHS NIH HHS/United States
- R01 AI043477/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous