Apc1638T: a mouse model delineating critical domains of the adenomatous polyposis coli protein involved in tumorigenesis and development - PubMed (original) (raw)
. 1999 May 15;13(10):1309-21.
doi: 10.1101/gad.13.10.1309.
M F Kielman, C Breukel, C Zurcher, K Neufeld, S Jagmohan-Changur, N Hofland, J van Dijk, R White, W Edelmann, R Kucherlapati, P M Khan, R Fodde
Affiliations
- PMID: 10346819
- PMCID: PMC316713
- DOI: 10.1101/gad.13.10.1309
Apc1638T: a mouse model delineating critical domains of the adenomatous polyposis coli protein involved in tumorigenesis and development
R Smits et al. Genes Dev. 1999.
Abstract
The adenomatous polyposis coli (APC) gene is considered as the true gatekeeper of colonic epithelial proliferation: It is mutated in the majority of colorectal tumors, and mutations occur at early stages of tumor development in mouse and man. These mutant proteins lack most of the seven 20-amino-acid repeats and all SAMP motifs that have been associated with down-regulation of intracellular beta-catenin levels. In addition, they lack the carboxy-terminal domains that bind to DLG, EB1, and microtubulin. APC also appears to be essential in development because homozygosity for mouse Apc mutations invariably results in early embryonic lethality. Here, we describe the generation of a mouse model carrying a targeted mutation at codon 1638 of the mouse Apc gene, Apc1638T, resulting in a truncated Apc protein encompassing three of the seven 20 amino acid repeats and one SAMP motif, but missing all of the carboxy-terminal domains thought to be associated with tumorigenesis. Surprisingly, homozygosity for the Apc1638T mutation is compatible with postnatal life. However, homozygous mutant animals are characterized by growth retardation, a reduced postnatal viability on the B6 genetic background, the absence of preputial glands, and the formation of nipple-associated cysts. Most importantly, Apc1638T/1638T animals that survive to adulthood are tumor free. Although the full complement of Apc1638T is sufficient for proper beta-catenin signaling, dosage reductions of the truncated protein result in increasingly severe defects in beta-catenin regulation. The SAMP motif retained in Apc1638T also appears to be important for this function as shown by analysis of the Apc1572T protein in which its targeted deletion results in a further reduction in the ability of properly controlling beta-catenin/Tcf signaling. These results indicate that the association with DLG, EB1, and microtubulin is less critical for the maintenance of homeostasis by APC than has been suggested previously, and that proper beta-catenin regulation by APC appears to be required for normal embryonic development and tumor suppression.
Figures
Figure 1
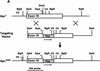
(A) Generation of the _Apc_1638T mutation. Schematic drawing of exon 15 of the mouse Apc gene, the targeting vector, and the resulting mutant allele. The PGK–hygromycin cassette was inserted at the _Sma_I site corresponding to codon 1638. Some of the restriction sites used for cloning or Southern analysis are shown. For the generation of the _Apc_1572T mutation, the PGK–hygromycin cassette was inserted between the same _Sma_I site and the _Ssp_I site corresponding to codon 1572. (B) Southern blot analysis of _Bgl_II and _Hin_dIII-digested DNA isolated from the various _Apc_-targeted ES clones, hybridized with a 3-kb fragment encompassing the 5′ half of exon 15. Molecular sizes of wild-type and mutant fragments are depicted. (Lane 1) Apc+/+; (lane 2) Apc+/1638T; (lane 3) Apc+/1572T; (lane 4) _Apc_1638N/1572T. (C) Western blot analysis of the various _Apc_-targeted ES clones. Molecular sizes of wild-type and mutant fragments are depicted. (Lane 1) Apc+/+; (lane 2) Apc+/1638T; (lane 3) _Apc_1638T/1638T; (lane 4) _Apc_1638N/1638T; (lane 5) _Apc_1638N/1638N; (lane 6) Apc+/1572T; (lane 7) _Apc_1638N/1572T. (D) Schematic representation of the 2843-amino-acid full-length Apc and the truncated 1638 and 1572-amino-acid polypeptides. The Apc1638T polypeptide lacks the carboxy-terminal domains that bind to tubulin, DLG, EB1-like proteins, and possibly p34cdc2. In addition, four of seven β-catenin down-regulating repeats and two of three conductin/axin-binding motifs are absent. The Apc1572T polypeptide also lacks the last remaining conductin/axin-binding motif.
Figure 1
(A) Generation of the _Apc_1638T mutation. Schematic drawing of exon 15 of the mouse Apc gene, the targeting vector, and the resulting mutant allele. The PGK–hygromycin cassette was inserted at the _Sma_I site corresponding to codon 1638. Some of the restriction sites used for cloning or Southern analysis are shown. For the generation of the _Apc_1572T mutation, the PGK–hygromycin cassette was inserted between the same _Sma_I site and the _Ssp_I site corresponding to codon 1572. (B) Southern blot analysis of _Bgl_II and _Hin_dIII-digested DNA isolated from the various _Apc_-targeted ES clones, hybridized with a 3-kb fragment encompassing the 5′ half of exon 15. Molecular sizes of wild-type and mutant fragments are depicted. (Lane 1) Apc+/+; (lane 2) Apc+/1638T; (lane 3) Apc+/1572T; (lane 4) _Apc_1638N/1572T. (C) Western blot analysis of the various _Apc_-targeted ES clones. Molecular sizes of wild-type and mutant fragments are depicted. (Lane 1) Apc+/+; (lane 2) Apc+/1638T; (lane 3) _Apc_1638T/1638T; (lane 4) _Apc_1638N/1638T; (lane 5) _Apc_1638N/1638N; (lane 6) Apc+/1572T; (lane 7) _Apc_1638N/1572T. (D) Schematic representation of the 2843-amino-acid full-length Apc and the truncated 1638 and 1572-amino-acid polypeptides. The Apc1638T polypeptide lacks the carboxy-terminal domains that bind to tubulin, DLG, EB1-like proteins, and possibly p34cdc2. In addition, four of seven β-catenin down-regulating repeats and two of three conductin/axin-binding motifs are absent. The Apc1572T polypeptide also lacks the last remaining conductin/axin-binding motif.
Figure 1
(A) Generation of the _Apc_1638T mutation. Schematic drawing of exon 15 of the mouse Apc gene, the targeting vector, and the resulting mutant allele. The PGK–hygromycin cassette was inserted at the _Sma_I site corresponding to codon 1638. Some of the restriction sites used for cloning or Southern analysis are shown. For the generation of the _Apc_1572T mutation, the PGK–hygromycin cassette was inserted between the same _Sma_I site and the _Ssp_I site corresponding to codon 1572. (B) Southern blot analysis of _Bgl_II and _Hin_dIII-digested DNA isolated from the various _Apc_-targeted ES clones, hybridized with a 3-kb fragment encompassing the 5′ half of exon 15. Molecular sizes of wild-type and mutant fragments are depicted. (Lane 1) Apc+/+; (lane 2) Apc+/1638T; (lane 3) Apc+/1572T; (lane 4) _Apc_1638N/1572T. (C) Western blot analysis of the various _Apc_-targeted ES clones. Molecular sizes of wild-type and mutant fragments are depicted. (Lane 1) Apc+/+; (lane 2) Apc+/1638T; (lane 3) _Apc_1638T/1638T; (lane 4) _Apc_1638N/1638T; (lane 5) _Apc_1638N/1638N; (lane 6) Apc+/1572T; (lane 7) _Apc_1638N/1572T. (D) Schematic representation of the 2843-amino-acid full-length Apc and the truncated 1638 and 1572-amino-acid polypeptides. The Apc1638T polypeptide lacks the carboxy-terminal domains that bind to tubulin, DLG, EB1-like proteins, and possibly p34cdc2. In addition, four of seven β-catenin down-regulating repeats and two of three conductin/axin-binding motifs are absent. The Apc1572T polypeptide also lacks the last remaining conductin/axin-binding motif.
Figure 1
(A) Generation of the _Apc_1638T mutation. Schematic drawing of exon 15 of the mouse Apc gene, the targeting vector, and the resulting mutant allele. The PGK–hygromycin cassette was inserted at the _Sma_I site corresponding to codon 1638. Some of the restriction sites used for cloning or Southern analysis are shown. For the generation of the _Apc_1572T mutation, the PGK–hygromycin cassette was inserted between the same _Sma_I site and the _Ssp_I site corresponding to codon 1572. (B) Southern blot analysis of _Bgl_II and _Hin_dIII-digested DNA isolated from the various _Apc_-targeted ES clones, hybridized with a 3-kb fragment encompassing the 5′ half of exon 15. Molecular sizes of wild-type and mutant fragments are depicted. (Lane 1) Apc+/+; (lane 2) Apc+/1638T; (lane 3) Apc+/1572T; (lane 4) _Apc_1638N/1572T. (C) Western blot analysis of the various _Apc_-targeted ES clones. Molecular sizes of wild-type and mutant fragments are depicted. (Lane 1) Apc+/+; (lane 2) Apc+/1638T; (lane 3) _Apc_1638T/1638T; (lane 4) _Apc_1638N/1638T; (lane 5) _Apc_1638N/1638N; (lane 6) Apc+/1572T; (lane 7) _Apc_1638N/1572T. (D) Schematic representation of the 2843-amino-acid full-length Apc and the truncated 1638 and 1572-amino-acid polypeptides. The Apc1638T polypeptide lacks the carboxy-terminal domains that bind to tubulin, DLG, EB1-like proteins, and possibly p34cdc2. In addition, four of seven β-catenin down-regulating repeats and two of three conductin/axin-binding motifs are absent. The Apc1572T polypeptide also lacks the last remaining conductin/axin-binding motif.
Figure 2
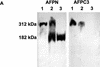
Protein analysis of the Apc1638T mutation. (A) Total protein lysates derived from brains of Apc+/+ (lane 1), Apc+/1638T (lane 2), and _Apc_1638T/1638T (lane 3) animals were either hybridized with AFPN (left) or AFPC3 (right). Similar results were obtained for liver and duodenum. (B) Apc+/+, Apc+/1638T, and _Apc_1638T/1638T ES cells were immunoprecipitated with either AFPN or AFPC3, and subsequently hybridized with APC (Ab-1) recognizing an amino-terminal epitope. In Apc+/1638T cells, immunoprecipitation of the full-length Apc with the AFPC3 antibody results in coprecipitation of the Apc1638T protein because of dimerization through the amino-terminal end of Apc. (C) The AFPN immunoprecipitations performed on the ES cells were hybridized with β-catenin and DLG monoclonals, showing that the Apc1638T protein is still capable of binding β-catenin, but does not associate with DLG.
Figure 2
Protein analysis of the Apc1638T mutation. (A) Total protein lysates derived from brains of Apc+/+ (lane 1), Apc+/1638T (lane 2), and _Apc_1638T/1638T (lane 3) animals were either hybridized with AFPN (left) or AFPC3 (right). Similar results were obtained for liver and duodenum. (B) Apc+/+, Apc+/1638T, and _Apc_1638T/1638T ES cells were immunoprecipitated with either AFPN or AFPC3, and subsequently hybridized with APC (Ab-1) recognizing an amino-terminal epitope. In Apc+/1638T cells, immunoprecipitation of the full-length Apc with the AFPC3 antibody results in coprecipitation of the Apc1638T protein because of dimerization through the amino-terminal end of Apc. (C) The AFPN immunoprecipitations performed on the ES cells were hybridized with β-catenin and DLG monoclonals, showing that the Apc1638T protein is still capable of binding β-catenin, but does not associate with DLG.
Figure 2
Protein analysis of the Apc1638T mutation. (A) Total protein lysates derived from brains of Apc+/+ (lane 1), Apc+/1638T (lane 2), and _Apc_1638T/1638T (lane 3) animals were either hybridized with AFPN (left) or AFPC3 (right). Similar results were obtained for liver and duodenum. (B) Apc+/+, Apc+/1638T, and _Apc_1638T/1638T ES cells were immunoprecipitated with either AFPN or AFPC3, and subsequently hybridized with APC (Ab-1) recognizing an amino-terminal epitope. In Apc+/1638T cells, immunoprecipitation of the full-length Apc with the AFPC3 antibody results in coprecipitation of the Apc1638T protein because of dimerization through the amino-terminal end of Apc. (C) The AFPN immunoprecipitations performed on the ES cells were hybridized with β-catenin and DLG monoclonals, showing that the Apc1638T protein is still capable of binding β-catenin, but does not associate with DLG.
Figure 3
β-Catenin/Tcf reporter assays performed in MEFs and ES cells of the depicted genotypes. Approximately 1 × 105 MEFs or 1 × 106 undifferentiated ES cells were transfected with either 0.75 μg of pTOPFLASH (shaded bars) or pFOPFLASH (solid bars) luciferase reporter construct, and cotransfected with 0.75 μg of CMV-galactosidase serving as an internal control (Korinek et al. 1997). All assays were performed as triplicate transfections as shown. pTOPFLASH/PFOPFLASH ratios are depicted for each cell line. ES cells homozygous for the _Apc_1638N mutation are clearly defective in β-catenin regulation, as demonstrated by an average 30-fold increase of the transcriptional activity of pTOPFLASH over its mutant pFOPFLASH control. In contrast, the Apc1638T protein appears to be functional both in MEFs and ES cells when expressed at wild-type levels. In _Apc_1638N/1638T ES cells haploinsufficient for the Apc1638T protein, a twofold increase of pTOPFLASH activity was observed. Apc1572T is more severly impaired in β-catenin down-regulation than Apc1638T, as demonstrated by an average twofold increase of pTOPFLASH activity in the _Apc_1638N/1572T ES line when compared with the _Apc_1638N/1638T clone.
Figure 4
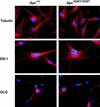
Immunolocalization of β-tubulin, EB-1, and DLG in Apc+/+ and Apc_1638T/1638T MEFs. Nuclei are counterstained with DAPI. Original magnification, 630_x.
Figure 5
The Apc1638T protein localizes to both membrane/cytoskeletal and nuclear cell fractions in _Apc_1638T/1638T MEFs. Following lysis and fractionation, equal amounts of total protein from the different fractions were analyzed by SDS-PAGE and immunoblotting with APC (Ab-1) antibody. The Apc1638T protein is present in the total sample (T), the membrane/cytoskeleton (M), and the nucleus (N) but not in the cytoplasm (C).
Figure 6
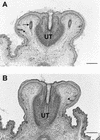
Hematoxylin and eosin-stained cross sections of the genital tubercle of a 18.5-day-old wild-type male embryo (A) and a homozygous _Apc_1638T male littermate (B). Two preputial gland anlagen (arrow) are clearly present in the mesenchyme adjacent to the urethra (UT) of the wild-type embryo, whereas they are completely absent at the corresponding position in the homozygous mutant embryo. In both embryos, primitive hair follicles (arrowheads) have formed with no apparent histological difference (Bar, 250 μm).
Figure 7
Histological appearance of a cutaneous cyst observed in a _Apc_1638T/1638T female. In this case, the predominantly infundibular epithelium of the cyst is in open connection with the skin surface. All Apc1638T cysts were present in the proximity of a nipple. Accordingly, some mammary ducts (MD) can be observed in the near vicinity of this cyst. Most of the other _Apc_1638T cysts were located deep in the dermis or in the subcutaneous fat layer above the panniculus carnosus, as it has been described for the _Apc_1638N mouse model (Smits et al. 1998). Section was stained with hematoxylin and eosin (Bar, 320 μm).
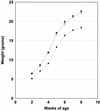
Figure 8
Growth curve of 35 _Apc_1638T/1638T mice (█) and their wild-type, (31, □) and heterozygous (88, ●) littermates. All mice were weighed on a weekly basis between 10 days and 8 weeks of age. Standard deviations are indicated as vertical bars. The average weights shown for each time point and genotype represent averages from both sexes. However, similar curves are obtained if the data are analyzed for each sex separately. Therefore, both male and female _Apc_1638T/1638T animals are characterized by a growth retardation.
Similar articles
- A Drosophila homolog of the tumor suppressor gene adenomatous polyposis coli down-regulates beta-catenin but its zygotic expression is not essential for the regulation of Armadillo.
Hayashi S, Rubinfeld B, Souza B, Polakis P, Wieschaus E, Levine AJ. Hayashi S, et al. Proc Natl Acad Sci U S A. 1997 Jan 7;94(1):242-7. doi: 10.1073/pnas.94.1.242. Proc Natl Acad Sci U S A. 1997. PMID: 8990193 Free PMC article. - The 'just-right' signaling model: APC somatic mutations are selected based on a specific level of activation of the beta-catenin signaling cascade.
Albuquerque C, Breukel C, van der Luijt R, Fidalgo P, Lage P, Slors FJ, Leitão CN, Fodde R, Smits R. Albuquerque C, et al. Hum Mol Genet. 2002 Jun 15;11(13):1549-60. doi: 10.1093/hmg/11.13.1549. Hum Mol Genet. 2002. PMID: 12045208 - A disturbance of intestinal epithelial cell population and kinetics in APC1638T mice.
Wang T, Onouchi T, Yamada NO, Matsuda S, Senda T. Wang T, et al. Med Mol Morphol. 2017 Jun;50(2):94-102. doi: 10.1007/s00795-016-0152-5. Epub 2017 Jan 9. Med Mol Morphol. 2017. PMID: 28070680 - [APC, beta-catenin, DLG].
Akiyama T. Akiyama T. Gan To Kagaku Ryoho. 1997 Sep;24(11):1432-5. Gan To Kagaku Ryoho. 1997. PMID: 9309137 Review. Japanese. - Adenomatous polyposis coli (Apc) tumor suppressor gene as a multifunctional gene.
Senda T, Shimomura A, Iizuka-Kogo A. Senda T, et al. Anat Sci Int. 2005 Sep;80(3):121-31. doi: 10.1111/j.1447-073x.2005.00106.x. Anat Sci Int. 2005. PMID: 16158975 Review.
Cited by
- Manipulation of DNA Repair Proficiency in Mouse Models of Colorectal Cancer.
Mcilhatton MA, Boivin GP, Groden J. Mcilhatton MA, et al. Biomed Res Int. 2016;2016:1414383. doi: 10.1155/2016/1414383. Epub 2016 Jun 20. Biomed Res Int. 2016. PMID: 27413734 Free PMC article. Review. - The canonical Wnt signalling pathway and its APC partner in colon cancer development.
Schneikert J, Behrens J. Schneikert J, et al. Gut. 2007 Mar;56(3):417-25. doi: 10.1136/gut.2006.093310. Epub 2006 Jul 13. Gut. 2007. PMID: 16840506 Free PMC article. Review. No abstract available. - Actin-dependent membrane association of the APC tumour suppressor in polarized mammalian epithelial cells.
Rosin-Arbesfeld R, Ihrke G, Bienz M. Rosin-Arbesfeld R, et al. EMBO J. 2001 Nov 1;20(21):5929-39. doi: 10.1093/emboj/20.21.5929. EMBO J. 2001. PMID: 11689433 Free PMC article. - Wnt/Beta-Catenin Signaling Regulation and a Role for Biomolecular Condensates.
Schaefer KN, Peifer M. Schaefer KN, et al. Dev Cell. 2019 Feb 25;48(4):429-444. doi: 10.1016/j.devcel.2019.01.025. Dev Cell. 2019. PMID: 30782412 Free PMC article. Review. - A targeted constitutive mutation in the APC tumor suppressor gene underlies mammary but not intestinal tumorigenesis.
Gaspar C, Franken P, Molenaar L, Breukel C, van der Valk M, Smits R, Fodde R. Gaspar C, et al. PLoS Genet. 2009 Jul;5(7):e1000547. doi: 10.1371/journal.pgen.1000547. Epub 2009 Jul 3. PLoS Genet. 2009. PMID: 19578404 Free PMC article.
References
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. - PubMed
- Behrens J, Jerchow B-A, Würtele M, Grimm J, Asbrand C, Wirtz R, Kühl M, Wedlich D, Birchmeier W. Functional interaction of an Axin homolog, Conductin, with β-Catenin, APC, and GSK3β. Science. 1998;280:596–599. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA09602/CA/NCI NIH HHS/United States
- CA76329-01/CA/NCI NIH HHS/United States
- R01 CA076329/CA/NCI NIH HHS/United States
- CA67944/CA/NCI NIH HHS/United States
- T32 CA009602/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous