Maintenance energy demand and starvation recovery dynamics of Nitrosomonas europaea and Nitrobacter winogradskyi cultivated in a retentostat with complete biomass retention - PubMed (original) (raw)
Maintenance energy demand and starvation recovery dynamics of Nitrosomonas europaea and Nitrobacter winogradskyi cultivated in a retentostat with complete biomass retention
W Tappe et al. Appl Environ Microbiol. 1999 Jun.
Abstract
Nitrosomonas europaea and Nitrobacter winogradskyi (strain "Engel") were grown in ammonia-limited and nitrite-limited conditions, respectively, in a retentostat with complete biomass retention at 25 degrees C and pH 8. Fitting the retentostat biomass and oxygen consumption data of N. europaea and N. winogradskyi to the linear equation for substrate utilization resulted in up to eight-times-lower maintenance requirements compared to the maintenance energy demand (m) calculated from chemostat experiments. Independent of the growth rate at different stages of such a retention culture, the maximum specific oxygen consumption rate measured by mass spectrometric analysis of inlet and outlet gas oxygen content always amounted to approximately 45 micromol of O2 mg-1 of biomass-C x h-1 for both N. europaea and N. winogradskyi. When bacteria were starved for different time periods (up to 3 months), the spontaneous respiratory activity after an ammonia or nitrite pulse decreased with increasing duration of the previous starvation time period, but the observed decrease was many times faster for N. winogradskyi than for N. europaea. Likewise, the velocity of resuscitation decreased with extended time periods of starvation. The increase in oxygen consumption rates during resuscitation referred to the reviving population only, since in parallel no significant increase in the cell concentrations was detectable. N. europaea more readily recovers from starvation than N. winogradskyi, explaining the occasionally observed nitrite accumulation in the environment after ammonia becomes available. From chloramphenicol (100 microg x ml-1) inhibition experiments with N. winogradskyi, it has been concluded that energy-starved cells must have a lower protein turnover rate than nonstarved cells. As pointed out by Stein and Arp (L. Y. Stein and D. J. Arp, Appl. Environ. Microbiol. 64:1514-1521, 1998), nitrifying bacteria in soil have to cope with extremely low nutrient concentrations. Therefore, a chemostat is probably not a suitable tool for studying their physiological properties during a long-lasting nutrient shortage. In comparison with chemostats, retentostats offer a more realistic approach with respect to substrate provision and availability.
Figures
FIG. 1
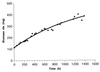
Biomass concentration in milligrams (dry weight) of N. europaea in continuous culture with 100% biomass retention (_r_s = 0.299 mmol of NH4+ h−1). Data show the increase of biomass after reaching 100 mg in the retentostat mode. The solid line represents the best fit for X t, a value obtained by using the equations 2 and 3 as described in Materials and Methods. The results of one experiment are shown.
FIG. 2
Cell concentration in cells per milliliter (triangles) and mean cell volume in cubic micrometers (circles) of N. winogradskyi in continuous culture with 100% biomass retention (_r_s = 1.42 mmol of NaNO2 h−1). Cells were batch grown during the first 80 h. From 1,100 h onwards, the biomass concentration remained at ca. 332 mg (dry weight) per liter. The ratio of _r_s to steady-state biomass concentration reflects a maintenance energy demand (m) of 4.3 μmol of NO2− per mg (dry weight) per hour. The results of the longest of three similar experiments are shown.
FIG. 3
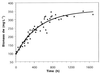
Biomass concentrations in milligrams (dry weight) per liter of three retentostat runs with N. winogradskyi (_r_s = 1.42 mmol of NaNO2− · h−1 in all runs). The data show the increase in biomass after it reached 100 mg (dry weight) per liter in the retentostat mode. The solid line represents the best fit. The fitted value for m was 2.8 μmol of NO2− per mg (dry weight) per h.
FIG. 4
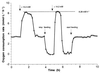
Oxygen consumption rate of N. winogradskyi responding to a pulse of 14.2 mM nitrite during continuous and disrupted feeding in a retentostat run with 100% biomass retention. The same specific maximum oxygen consumption rate of 44 μmol O2 · mg of biomass-C−1 · h−1 was reached after the substrate pulse in both cases at a biomass concentration of 188 mg of biomass-C · liter−1.
FIG. 5
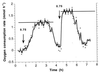
Resuscitation of N. winogradskyi after 3 days of nitrite depletion. Definitions: ↓ = nitrite pulses of 14.2 mM; biomass concentration = 137 mg of biomass-C · liter−1; maximum oxygen consumption rates 30 (a), 33 (b), 36 (c), and 44 μmol O2 · mg of biomass-C−1 · h−1. The maximum activity (see Fig. 4) was reestablished at the latest after 12 h of continuous feeding and the fourth pulse.
FIG. 6
Resuscitation of N. europaea after 17 days of ammonia depletion. Definitions: ↓ = ammonia pulses of 0.75 mM; biomass concentration = 138 mg of biomass-C · liter−1; maximum oxygen consumption rate of the first pulse = 22 μmol of O2 · mg of biomass-C−1 · h−1.
FIG. 7
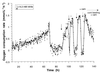
Resuscitation of N. winogradskyi after 35 days of nitrite depletion. With the last pulse, the maximum specific activity amounted to 50% of the activity of unstarved cells (Fig. 4). Together with the last pulse, chloramphenicol was added to give a final concentration of 100 μg · ml−1. At the same time, the substrate supply was switched to continuous feeding, also including chloramphenicol at 100 μg · ml−1 to maintain the concentration. (Note that the absolute activity is lower compared to Fig. 4 and 5 due to the removal of biomass for another experiment.)
Similar articles
- Axenic cultures of Nitrosomonas europaea and Nitrobacter winogradskyi in autotrophic conditions: a new protocol for kinetic studies.
Farges B, Poughon L, Roriz D, Creuly C, Dussap CG, Lasseur C. Farges B, et al. Appl Biochem Biotechnol. 2012 Jul;167(5):1076-91. doi: 10.1007/s12010-012-9651-6. Epub 2012 Mar 27. Appl Biochem Biotechnol. 2012. PMID: 22451350 - Interactions of Nitrosomonas europaea and Nitrobacter winogradskyi grown in co-culture.
Pérez J, Buchanan A, Mellbye B, Ferrell R, Chang JH, Chaplen F, Bottomley PJ, Arp DJ, Sayavedra-Soto LA. Pérez J, et al. Arch Microbiol. 2015 Jan;197(1):79-89. doi: 10.1007/s00203-014-1056-1. Epub 2014 Nov 2. Arch Microbiol. 2015. PMID: 25362506 - Effects of Bacterial Community Members on the Proteome of the Ammonia-Oxidizing Bacterium Nitrosomonas sp. Strain Is79.
Sedlacek CJ, Nielsen S, Greis KD, Haffey WD, Revsbech NP, Ticak T, Laanbroek HJ, Bollmann A. Sedlacek CJ, et al. Appl Environ Microbiol. 2016 Jul 15;82(15):4776-4788. doi: 10.1128/AEM.01171-16. Print 2016 Aug 1. Appl Environ Microbiol. 2016. PMID: 27235442 Free PMC article. - Energy model and metabolic flux analysis for autotrophic nitrifiers.
Poughon L, Dussap CG, Gros JB. Poughon L, et al. Biotechnol Bioeng. 2001 Feb 20;72(4):416-33. Biotechnol Bioeng. 2001. PMID: 11180062 Review. - Strategies of aerobic ammonia-oxidizing bacteria for coping with nutrient and oxygen fluctuations.
Geets J, Boon N, Verstraete W. Geets J, et al. FEMS Microbiol Ecol. 2006 Oct;58(1):1-13. doi: 10.1111/j.1574-6941.2006.00170.x. FEMS Microbiol Ecol. 2006. PMID: 16958903 Review.
Cited by
- Sulfur isotope enrichment during maintenance metabolism in the thermophilic sulfate-reducing bacterium Desulfotomaculum putei.
Davidson MM, Bisher ME, Pratt LM, Fong J, Southam G, Pfiffner SM, Reches Z, Onstott TC. Davidson MM, et al. Appl Environ Microbiol. 2009 Sep;75(17):5621-30. doi: 10.1128/AEM.02948-08. Epub 2009 Jun 26. Appl Environ Microbiol. 2009. PMID: 19561180 Free PMC article. - Effect of starvation and chloramphenicol on acceleration of bacterial dihexyl sulfosuccinate biotransformation.
Chmelárová Z, Závadská I, Húska J, Tóth D. Chmelárová Z, et al. Folia Microbiol (Praha). 2000;45(6):493-5. doi: 10.1007/BF02818716. Folia Microbiol (Praha). 2000. PMID: 11501413 - Quantitative physiology of Saccharomyces cerevisiae at near-zero specific growth rates.
Boender LG, de Hulster EA, van Maris AJ, Daran-Lapujade PA, Pronk JT. Boender LG, et al. Appl Environ Microbiol. 2009 Sep;75(17):5607-14. doi: 10.1128/AEM.00429-09. Epub 2009 Jul 10. Appl Environ Microbiol. 2009. PMID: 19592533 Free PMC article. - Microbial life under extreme energy limitation.
Hoehler TM, Jørgensen BB. Hoehler TM, et al. Nat Rev Microbiol. 2013 Feb;11(2):83-94. doi: 10.1038/nrmicro2939. Nat Rev Microbiol. 2013. PMID: 23321532 Review. - Sulfur mass-independent fractionation in subsurface fracture waters indicates a long-standing sulfur cycle in Precambrian rocks.
Li L, Wing BA, Bui TH, McDermott JM, Slater GF, Wei S, Lacrampe-Couloume G, Lollar BS. Li L, et al. Nat Commun. 2016 Oct 27;7:13252. doi: 10.1038/ncomms13252. Nat Commun. 2016. PMID: 27807346 Free PMC article.
References
- Arbige M, Chesbro W R. Very slow growth of Bacillus polymyxa: stringent response and maintenance energy. Arch Microbiol. 1982;132:338–344.
- Azov Y, Tregubova T. Nitrification processes in stabilisation reservoirs. Water Sci Technol. 1995;31:313–319.
- Bakken L R. Culturable and nonculturable bacteria in soil. In: van Elsas J D, Trevors J T, Wellington E M H, editors. Modern soil microbiology. New York, N.Y: Marcel Dekker; 1997. pp. 47–61.
- Balmelle B, Nguyen K M, Capdeville B, Cornier J C, Deguin A. Study of factors controlling nitrite build-up in biological processes for water nitrification. Water Sci Technol. 1992;26:1017–1025.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous