Saccharomyces cerevisiae mid2p is a potential cell wall stress sensor and upstream activator of the PKC1-MPK1 cell integrity pathway - PubMed (original) (raw)
Saccharomyces cerevisiae mid2p is a potential cell wall stress sensor and upstream activator of the PKC1-MPK1 cell integrity pathway
T Ketela et al. J Bacteriol. 1999 Jun.
Abstract
The MID2 gene of Saccharomyces cerevisiae encodes a protein with structural features indicative of a plasma membrane-associated cell wall sensor. MID2 was isolated as a multicopy activator of the Skn7p transcription factor. Deletion of MID2 causes resistance to calcofluor white, diminished production of stress-induced cell wall chitin under a variety of conditions, and changes in growth rate and viability in a number of different cell wall biosynthesis mutants. Overexpression of MID2 causes hyperaccumulation of chitin and increased sensitivity to calcofluor white. alpha-Factor hypersensitivity of mid2Delta mutants can be suppressed by overexpression of upstream elements of the cell integrity pathway, including PKC1, RHO1, WSC1, and WSC2. Mid2p and Wsc1p appear to have overlapping roles in maintaining cell integrity since mid2Delta wsc1Delta mutants are inviable on medium that does not contain osmotic support. A role for MID2 in the cell integrity pathway is further supported by the finding that MID2 is required for induction of Mpk1p tyrosine phosphorylation during exposure to alpha-factor, calcofluor white, or high temperature. Our data are consistent with a role for Mid2p in sensing cell wall stress and in activation of a response that includes both increased chitin synthesis and the Mpk1p mitogen-activated protein kinase cell integrity pathway. In addition, we have identified an open reading frame, MTL1, which encodes a protein with both structural and functional similarity to Mid2p.
Figures
FIG. 1
Multicopy MID2 activates Skn7p-LexA-dependent transcription of HIS3, which allows growth on medium lacking histidine. Reporter strains containing Skn7p-LexA and either pRS425 (TK60) or pRS425-MID2 (TK61) were grown on selective medium lacking histidine and containing 30 mM 3AT.
FIG. 2
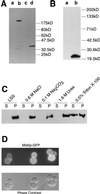
Cell biology of Mid2p. (A) Immunoblot analysis of cell extracts from TK82 (vector only) (lane a), TK84 (MID2-HA) (lane b), _pmt1Δ pmt2_Δ (vector only) (lane c), and _pmt1Δ pmt2_Δ (MID2-HA). (B) Immunoblot analysis of cell extracts from TK82 (vector only) (lane a) and TK85 (ΔS/T-Mid2p-HA) (lane b). (C) Immunoblot analysis of cell fractions from TK84 to demonstrate membrane association of Mid2p. LSS, low-speed-spin pellet fraction. (D) In cells expressing pRS426-MID2-GFP (TK98), Mid2p-GFP is localized to the cell periphery.
FIG. 3
Deletion of MID2 has effects on growth of different cell wall mutants. (A) Representative tetratype tetrad from TK101 (_kre6Δ mid2_Δ heterozygous diploid). (B) Representative tetratype tetrad from TK102 (_kre9Δ mid2_Δ heterozygous diploid). (C) Single cells containing the indicated plasmids were placed on selective agar and grown at 30°C for 4 days. Photo is representative of effect seen in three isolates each of three transformations. (D) Representative tetratype tetrad from TK103 (_fks1Δ mid2_Δ heterozygous diploid).
FIG. 4
Dosage of MID2 affects sensitivity to calcofluor white. Mid-log-phase cells were diluted to a concentration of 3 × 106 cells/ml; 5 μl of this suspension and three subsequent 10-fold serial dilutions were each spotted onto the indicated medium. (A) SEY6210a (wild type) (row a) and TK88 (_mid2_Δ) (row b) cells were spotted onto YEPD containing 0 and 20 μg of calcofluor white (CFW) per ml. (B) TK82 [wild type (pRS426)] (row a), TK83 [wild type (pRS426-MID2)] (row b), TK86 [wild type (pVT101U)] (row c), and TK87 [wild type (pVT101U-MID2)] (row d) were spotted on uracil dropout medium containing 0 and 2.5 μg of calcofluor white per ml. (C) TK86 (row a), TK87 (row b), TK99 [_chs3_Δ (pVT101U)] (row c), and TK100 [_chs3_Δ (pVT101U-MID2)] (row d) were spotted onto uracil dropout medium containing 0, 2.5, and 15 μg of calcofluor white per ml.
FIG. 5
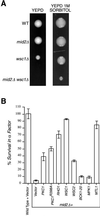
Genetic interactions between MID2 and members of the cell integrity pathway. (A) Representative tetratype tetrads of TK104 (_mid2Δ wsc1_Δ heterozygous diploid) dissected onto either YEPD or YEPD plus 1 M sorbitol. YEPD plates were incubated for 60 h at 30°C, YEPD–1 M sorbitol plates were incubated for 80 h at 30°C. WT, wild type. (B) Members of the cell integrity pathway suppress α-factor-induced death in _mid2_Δ mutants. Wild-type cells containing pRS426 vector only and _mid2_Δ mutants carrying pRS426, YEP13-PKC1, pBM743-PKC1R398A (GAL-driven, hyperactive PKC1), pRS426-RHO1, pRS426-WSC1, pRS426-WSC2, pRS316-BCK1-20 (hyperactive BCK1), YEP352-MPK1, and pRS425-MTL1 in liquid medium were exposed to α-factor. Percentage of survival was measured by spreading liquid medium before and after α-factor exposure (330 mn) on petri dishes and counting colonies derived from single cells.
FIG. 6
Immunoblot analysis of Mpk1p-HA tyrosine phosphorylation. Lanes are loaded with equal amounts of extracts from strains TK96 [wild type (pFL44)] (lanes a), TK97 [wild type (pFL44-MPK1-HA)] (lanes b), TK93 [_mid2_Δ (pFL44)] (lanes c), and TK94 [_mid2_Δ(pFL44-MPK1-HA)] (lanes d). Cultures exposed to α-factor (A), calcofluor white (B), or high-temperature growth (C) were harvested at the indicated times, and total cell proteins were subject to SDS-PAGE and Western blotting. In the top panel of each pair, tyrosine phosphorylation of Mpk1p-HA is detected by antiphosphotyrosine antibody 4G10. In the second panel of each pair, equal loading of Mpk1p-HA is demonstrated by anti-HA antibody HA11.
FIG. 7
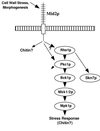
Model of Mid2p activity. Mid2p responds to cell wall stress by activating the cell integrity pathway and increasing chitin synthesis.
Similar articles
- A synthetic analysis of the Saccharomyces cerevisiae stress sensor Mid2p, and identification of a Mid2p-interacting protein, Zeo1p, that modulates the PKC1-MPK1 cell integrity pathway.
Green R, Lesage G, Sdicu AM, Ménard P, Bussey H. Green R, et al. Microbiology (Reading). 2003 Sep;149(Pt 9):2487-2499. doi: 10.1099/mic.0.26471-0. Microbiology (Reading). 2003. PMID: 12949174 - Cell wall perturbation in yeast results in dual phosphorylation of the Slt2/Mpk1 MAP kinase and in an Slt2-mediated increase in FKS2-lacZ expression, glucanase resistance and thermotolerance.
de Nobel H, Ruiz C, Martin H, Morris W, Brul S, Molina M, Klis FM. de Nobel H, et al. Microbiology (Reading). 2000 Sep;146 ( Pt 9):2121-2132. doi: 10.1099/00221287-146-9-2121. Microbiology (Reading). 2000. PMID: 10974100 - Saccharomyces cerevisiae heat shock transcription factor regulates cell wall remodeling in response to heat shock.
Imazu H, Sakurai H. Imazu H, et al. Eukaryot Cell. 2005 Jun;4(6):1050-6. doi: 10.1128/EC.4.6.1050-1056.2005. Eukaryot Cell. 2005. PMID: 15947197 Free PMC article. - Together we are strong--cell wall integrity sensors in yeasts.
Rodicio R, Heinisch JJ. Rodicio R, et al. Yeast. 2010 Aug;27(8):531-40. doi: 10.1002/yea.1785. Yeast. 2010. PMID: 20641024 Review. - Cell wall integrity signaling in Saccharomyces cerevisiae.
Levin DE. Levin DE. Microbiol Mol Biol Rev. 2005 Jun;69(2):262-91. doi: 10.1128/MMBR.69.2.262-291.2005. Microbiol Mol Biol Rev. 2005. PMID: 15944456 Free PMC article. Review.
Cited by
- A framework for mapping, visualisation and automatic model creation of signal-transduction networks.
Tiger CF, Krause F, Cedersund G, Palmér R, Klipp E, Hohmann S, Kitano H, Krantz M. Tiger CF, et al. Mol Syst Biol. 2012 Apr 24;8:578. doi: 10.1038/msb.2012.12. Mol Syst Biol. 2012. PMID: 22531118 Free PMC article. - Systems Level Analysis of the Yeast Osmo-Stat.
Talemi SR, Tiger CF, Andersson M, Babazadeh R, Welkenhuysen N, Klipp E, Hohmann S, Schaber J. Talemi SR, et al. Sci Rep. 2016 Aug 12;6:30950. doi: 10.1038/srep30950. Sci Rep. 2016. PMID: 27515486 Free PMC article. - A two-hybrid screen of the yeast proteome for Hsp90 interactors uncovers a novel Hsp90 chaperone requirement in the activity of a stress-activated mitogen-activated protein kinase, Slt2p (Mpk1p).
Millson SH, Truman AW, King V, Prodromou C, Pearl LH, Piper PW. Millson SH, et al. Eukaryot Cell. 2005 May;4(5):849-60. doi: 10.1128/EC.4.5.849-860.2005. Eukaryot Cell. 2005. PMID: 15879519 Free PMC article. - The temperature-sensitive role of Cryptococcus neoformans ROM2 in cell morphogenesis.
Fuchs BB, Tang RJ, Mylonakis E. Fuchs BB, et al. PLoS One. 2007 Apr 11;2(4):e368. doi: 10.1371/journal.pone.0000368. PLoS One. 2007. PMID: 17426816 Free PMC article. - Mutations that are synthetically lethal with a gas1Delta allele cause defects in the cell wall of Saccharomyces cerevisiae.
Tomishige N, Noda Y, Adachi H, Shimoi H, Takatsuki A, Yoda K. Tomishige N, et al. Mol Genet Genomics. 2003 Jul;269(4):562-73. doi: 10.1007/s00438-003-0864-9. Epub 2003 Jun 25. Mol Genet Genomics. 2003. PMID: 12827498
References
- Alberts A S, Bouquin N, Johnston L H, Treisman R. Analysis of RhoA-binding proteins reveals an interaction domain conserved in heterotrimeric G protein beta subunits and the yeast response regulator protein Skn7. J Biol Chem. 1998;273:8616–8622. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases