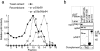The unstable F-box protein p58-Ctf13 forms the structural core of the CBF3 kinetochore complex - PubMed (original) (raw)
The unstable F-box protein p58-Ctf13 forms the structural core of the CBF3 kinetochore complex
I D Russell et al. J Cell Biol. 1999.
Abstract
Kinetochores are smaller and more accessible experimentally in budding yeast than in any other eukaryote. Believing that simple and complex kinetochores have important structural and functional properties in common, we characterized the structure of CBF3, the essential centromere-binding complex that initiates kinetochore formation in Saccharomyces cerevisiae. We find that the four subunits of CBF3 are multimeric in solution: p23(Skp1) and p58(Ctf13) form a heterodimer, and p64(Cep3) and p110(Ndc10) form homodimers. Subcomplexes involving p58 and each of the other CBF3 subunits can assemble in the absence of centromeric DNA. In these subcomplexes, p58 appears to function as a structural core mediating stable interactions among other CBF3 proteins. p58 has a short half-life in yeast, being subject to ubiquitin-dependent proteolysis, but we find that it is much more stable following association with p64. We propose that p23(Skp1)-p58-p64 complexes constitute the primary pool of active p58 in yeast cells. These complexes can either dissociate, reexposing p58 to the degradation pathway, or can bind to p110 and centromeric DNA, forming a functional CBF3 complex in which p58 is fully protected from degradation. This pathway may constitute an editing mechanism preventing the formation of ectopic kinetochores and ensuring the fidelity of chromosome segregation.
Figures
Figure 1
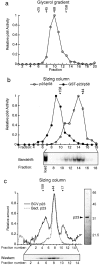
Hydrodynamic analysis of p110. (a) Purified p110 was applied to Superose 6 gel filtration columns and the eluate analyzed by absorption at 280 nm (dotted line), by bandshift complementation (solid line), and by Western blotting (gel). Arrows indicate the peak elution volumes for the molecular mass standards. For the complementation assay, excess p23Skp1, p58, p64, and radiolabeled CDEIII-containing probe were added and DNA-protein complexes were detected on bandshift gels. For the Western blot, fractions were TCA-precipitated, separated by SDS-PAGE, and immunoblotted using polyclonal rabbit anti-p110 antibodies and 125I-labeled protein A. A Coomassie-stained gel of recombinant p110 is shown to the right of the graph. The upper band is full-length p110 and the lower band is a p110 breakdown product, as determined by protein sequencing. (b) Western blot of p110 analyzed on a 15–35% glycerol gradient. The positions on the gradient of the protein standards are indicated above the gel panel. (c) Data and standard curves for three independent chromatographic separations or (d) glycerol gradient analyses. The data points show the positions of protein standards (see Materials and Methods) and the arrow shows the elution volume of p110. (e) Pull-down experiment demonstrating that p110 self-associates. GST-p110 or GST was expressed in insect cells alone or in combination with native p110, protein lysates were prepared, and GST protein complexes were isolated on glutathione-Sepharose beads. Bead-bound proteins were separated on 10% SDS-PAGE and immunoblotted with anti-p110 antibodies.
Figure 2
Hydrodynamic analysis and domain mapping of p64. (a) Purified p64 was applied to a Superose 12 gel filtration column and the eluate analyzed by absorption at 280 nm (dotted line), by bandshift complementation (solid line), and by Western blotting (gel; see Fig. 1 for details). To the right of the graph, a Coomassie-stained gel of recombinant p64 shows that it is present as a single pure species. (b) Western blot of p64 analyzed on a 15– 35% glycerol gradient. Hydrodynamic data were obtained from three independent experiments, as described in Fig. 1. (c) Pull-down experiment demonstrating that p64 self-associates. GSTp64 or GST were expressed in insect cells either alone or in combination with tagged p64 (carrying an amino-terminal MRGSHis6 tag; Qiagen), protein lysates were prepared, and GST protein complexes were isolated using glutathione-Sepharose. Bead-bound proteins were separated on 10% SDS-PAGE and immunoblotted with anti-MRGSHis6 tag antibodies. (d) Coomassie-stained gel of purified bacterially expressed p64 treated with limiting amounts of proteolytic enzymes to identify hypersensitive sites and delineate physical domains. The bacterially expressed p64 used in this experiment and the insect cell–expressed p64 analyzed in a and b, have identical specific activities for DNA binding (data not shown). 0.2 mg/ml p64 (lane 1) was treated at 30°C with 5 μg/ml trypsin for 10 min (lane 2), 20 min (lane 3), 40 min (lane 4), or 80 min (lane 5) or with 1 μg/ml chymotrypsin (lane 6) or 2 μg/ml V8 protease (lane 7) for 80 min. Digestions were stopped by boiling samples in SDS-PAGE loading buffer that contained PMSF. (e) The amino-terminal 100 residues of p64 are shown and the sites of tryptic cleavage for the fragments marked with arrows in c are indicated. The tryptic cleavage sites were identified by NH2-terminal microsequencing. The Zn2Cys6 zinc cluster domain of p64 (boxed) contains multiple potential trypsin recognition sequences (basic amino acids, shaded). No major fragments with cleavage sites within the zinc cluster were recovered, suggesting that the zinc cluster is indeed a compact folded structure. (f) Competition analysis on bandshift gels of wild-type (WT) and mutant 88-bp CDEIII DNA sequences. CBF3 and radiolabeled probe were mixed with a 4–200-fold excess of unlabeled centromeric competitor DNAs (as indicated) and analyzed on bandshift gels. Values are expressed as a percentage of the binding observed in the absence of competitor. Error bars show the mean and range of independent determinations. (g) Sequences of the various competitor DNAs showing only a relevant 21-bp segment of the 88-bp probes. Multiple point mutations in mutants 1 and 2 were introduced using PCR, and the 3-bp deletion in mutant 3 introduced as previously described (Espelin et al., 1997).
Figure 2
Hydrodynamic analysis and domain mapping of p64. (a) Purified p64 was applied to a Superose 12 gel filtration column and the eluate analyzed by absorption at 280 nm (dotted line), by bandshift complementation (solid line), and by Western blotting (gel; see Fig. 1 for details). To the right of the graph, a Coomassie-stained gel of recombinant p64 shows that it is present as a single pure species. (b) Western blot of p64 analyzed on a 15– 35% glycerol gradient. Hydrodynamic data were obtained from three independent experiments, as described in Fig. 1. (c) Pull-down experiment demonstrating that p64 self-associates. GSTp64 or GST were expressed in insect cells either alone or in combination with tagged p64 (carrying an amino-terminal MRGSHis6 tag; Qiagen), protein lysates were prepared, and GST protein complexes were isolated using glutathione-Sepharose. Bead-bound proteins were separated on 10% SDS-PAGE and immunoblotted with anti-MRGSHis6 tag antibodies. (d) Coomassie-stained gel of purified bacterially expressed p64 treated with limiting amounts of proteolytic enzymes to identify hypersensitive sites and delineate physical domains. The bacterially expressed p64 used in this experiment and the insect cell–expressed p64 analyzed in a and b, have identical specific activities for DNA binding (data not shown). 0.2 mg/ml p64 (lane 1) was treated at 30°C with 5 μg/ml trypsin for 10 min (lane 2), 20 min (lane 3), 40 min (lane 4), or 80 min (lane 5) or with 1 μg/ml chymotrypsin (lane 6) or 2 μg/ml V8 protease (lane 7) for 80 min. Digestions were stopped by boiling samples in SDS-PAGE loading buffer that contained PMSF. (e) The amino-terminal 100 residues of p64 are shown and the sites of tryptic cleavage for the fragments marked with arrows in c are indicated. The tryptic cleavage sites were identified by NH2-terminal microsequencing. The Zn2Cys6 zinc cluster domain of p64 (boxed) contains multiple potential trypsin recognition sequences (basic amino acids, shaded). No major fragments with cleavage sites within the zinc cluster were recovered, suggesting that the zinc cluster is indeed a compact folded structure. (f) Competition analysis on bandshift gels of wild-type (WT) and mutant 88-bp CDEIII DNA sequences. CBF3 and radiolabeled probe were mixed with a 4–200-fold excess of unlabeled centromeric competitor DNAs (as indicated) and analyzed on bandshift gels. Values are expressed as a percentage of the binding observed in the absence of competitor. Error bars show the mean and range of independent determinations. (g) Sequences of the various competitor DNAs showing only a relevant 21-bp segment of the 88-bp probes. Multiple point mutations in mutants 1 and 2 were introduced using PCR, and the 3-bp deletion in mutant 3 introduced as previously described (Espelin et al., 1997).
Figure 3
Probing the binding of p23Skp1 to p58. (a) 5–25% glycerol gradient of extract from cells coexpressing p58 and p23Skp1 analyzed using a bandshift complementation assay for p58. (b) Extracts from baculovirus-infected insect cells coexpressing p58 and p23Skp1 (open circles) or p58 and GST p23Skp1 (solid circles) were applied to a Superose 12 gel filtration column and the eluate analyzed using a bandshift complementation assay. The resulting bandshift complementation gel of the p58/p23Skp1 extract is shown below. (c) Purified p23Skp1 expressed in either insect cells or bacteria was applied to a Superose 12 gel filtration column and the eluate analyzed by absorption at 280 nm (dotted line) and by Western blotting (solid line; see Fig. 1 for details). To the right of the graph, a Coomassie-stained gel of recombinant p23 shows that it is present as a single pure species. Because activity assays for p23Skp1 are nonquantitative, no activity plot is shown.
Figure 4
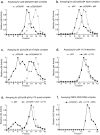
Associations among CBF3 proteins. Insect cell extracts containing CBF3 proteins were applied to Superose 12 gel filtration columns and assayed for p58 activity (a and c) or for p64 activity (b, d, and e) using bandshift complementation assays. In these samples, the total amount of insect cell lysate was kept constant to ensure that changes in apparent molecular mass arose from interactions among CBF3 proteins and not as a consequence of binding to contaminating insect proteins. (a) Elution profile of p58 activity from a column to which extract containing p23Skp1, p58, and p64 (solid line) or, for comparison, extract containing only p23Skp1 and p58 (dotted line), was applied. (b) Elution profile of p64 activity from a column to which a mixture of extracts from cells coexpressing p23Skp1 and p58 and cells expressing p64 (solid line) or, for comparison, extract containing only p64 (dotted line), was applied. (c) Elution profile of p58 activity from a column to which extract containing p23Skp1, p58, and p110 (solid line) or, for comparison, extract containing p23Skp1 and p58 only (dotted line), was applied. (d) Elution profile of p64 activity from a column to which a mixture of extracts from cells expressing p64 and cells expressing p110 (solid line) or, for comparison, extract containing p64 alone (dotted line) was applied. (e) p64 activity in an eluate from a Superose 12 column to which a mixture of extracts from cells expressing p64, cells expressing p110, and cells coexpressing p23Skp1 and p58 was applied (solid line). For comparison, a p64 activity profile is shown (dotted lines) for a column to which only the p64-containing extract was applied. The shift of p64 activity to fraction 5 represents the formation of a multiprotein complex likely containing all four CBF3 subunits (see text for details). (f) Profile of the sedimentation of CBF3-CDEIII complexes in a 15–35% glycerol gradient. CBF3-DNA complexes were generated by incubating extracts from insect cells coexpressing p23Skp1, p58, p64, and p110 with radiolabeled 56 base CDEIII probe. Fractions from the gradient were analyzed by loading them directly onto bandshift gels.
Figure 5
Mapping p58 sequences required for CBF3 interactions. (a) In vitro binding assay. In vitro translated 35S-p58 was incubated with either GSTp23Skp1, GSTp64, GST-p110, or GST alone immobilized on glutathione-Sepharose beads. The beads were spun down, washed and the bead-bound 35S-p58 was detected by SDS-PAGE and autoradiography. Fourfold more p58 was incubated with the beads (lanes 2–5) than was run as the input (lane 1). The experiment in lane 5 was run separately but is scaled to match the exposure of lanes 1–4. (b) Fragments of p58 were translated in vitro in reticulocyte lysates and tested for their ability to bind to GSTp23Skp1 or GSTp64 expressed in bacteria or to GSTp110 expressed in yeast. Due to variations in the efficiency with which different p58 fragments were translated, the amount of bound p58 was normalized to the amount of input p58 and the results are depicted as follows: +++, >25% bound; ++, 11–25% bound; +, 5–10% bound; and −, <5% bound.
Figure 6
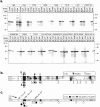
p58 contains a functional F-box that is required for binding to p23Skp1. (a) p58 variants containing point mutations in the F-box were translated in rabbit reticulocyte lysates and assayed for their ability to bind to GSTp23Skp1, GSTp64, or GST alone using the in vitro binding assay described in Fig. 1 a. (b) Alignment of p58 with the F-box proteins Cdc4p and Cyclin F. The consensus residues in the F-box are indicated by the dark (highly conserved) or gray (somewhat conserved) boxes. Note that not all of the “consensus” residues are found in the p58 F-box (p58-Y21 for example is in a conserved position, but does not match the consensus leucine). Black arrows indicate point mutations that abolished p58/p23Skp1 interactions in vitro and white arrows indicate point mutations that had no effect (as shown in a). Brackets and the A/A notation indicate the introduction of a double point mutation (L12A/P13A) into p58. p58 mutations are indicated below the alignment and Cdc4p mutations above. (c) Sequence of p58 showing the positions of F-box mutants that were tested for their ability to function in vivo. Mutant alleles inviable in vivo are marked with black circles (L12D, L12A-P13A, Y21R), alleles that exhibited wild-type growth in vivo are marked with white circles (P13A, K18A/E19A, V20A, L24A, D25A, D36A, K40A/D43A, E45A/K49A, K53A/R54A, K56A/R57A, and K59A/K60A), and a single allele that exhibited slow growth is marked with a black/white circle (L12A). From the class of mutants that were viable but showed no p58-p23Skp1 interaction in vitro, the L12A and V20A were selected for further analysis. The rate of loss of a nonessential tester chromosome (per generation) is shown for these mutant proteins and for a control wild-type strain.
Figure 7
Probing p58 interactions in yeast. (a) p58 activity in eluates from a Superose 12 column to which extracts from p23Skp1/p58 coexpressing insect cells (open circles), p23Skp1/ p58/p64 coexpressing insect cells (open squares), or wild-type yeast cells (solid line) were applied. The peak of p58 activity in the yeast extract overlaps the peak of the recombinant p23Skp1-p58-p64 co-complex. (b) Full-length or truncated p58 was fused to GST and expressed from the GAL1 promoter in yeast. GSTp58 and associated proteins were purified using glutathione-Sepharose, and the Sepharose-bound proteins analyzed by immunoblotting using anti-p23Skp1, anti-p64 or anti-GST as indicated (lanes 1–4). A similar analysis of proteins bound to GSTp110 was performed as a negative control (lane 5). The ability of each p58 fragment or mutant to complement a p58 deletion in vivo is indicated in the bottom panel. Unfused p58(L12A/P13A) protein (note 1) was unable to complement function in vivo; the GSTp58(L12A/P13A) fusion protein was not tested.
Figure 8
GSTp58 is stabilized in vivo by the overexpression of p64 but not p110 or p23Skp1. (a) Promoter shut-off experiments were used to compare the stability of GSTp58 (squares) to GST alone (circles). Yeast cells containing plasmid borne GAL1-GSTp58 and GAL1-GST were grown in galactose media, a sample was chilled and reserved for time 0, and the remaining cells were washed into glucose media, grown at 30°C, and aliquots removed to ice at 30 and 90 min following addition of glucose. Extracts were prepared and GST fused proteins purified on glutathione-Sepharose from 2 mg of total yeast protein by binding to glutathione-Sepharose. Bead-bound GST fusions were loaded in duplicate lanes on SDS-containing gels and GST proteins were detected by immunoblotting with an anti-GST antibody and 125I-labeled protein A. The rate of degradation of HA-tagged p58 (HAp58) is also plotted for comparison (diamonds; see Kaplan et al., 1997). (b) Stability of GSTp58 in a wild-type strain (circles) or a strain containing an integrated copy of GAL1-p64 (squares) as determined by promoter shut-off. (c) Stability of GSTp58 in a strain containing an integrated copy of GAL1-p23 (squares) or GAL1-p110 (circles). (d) Stability of the GAL1-GSTp58(Δ139–336) proteins, which does not bind to p64, in a wild-type strain (circles) or a strain containing an integrated copy of GAL1-p64 (squares). (e) Rate of degradation of GST fusions to an internal deletion of p58 lacking a putative PEST sequence (Δ211–230; circles), to p58 carrying a double point mutation in the F-box (L12A/P13A, squares), (f) to the p58 F-box (1–58; circles) or to an NH2-terminal truncation of p58 lacking the F-box (139–479; diamonds). Each data point represents the average of several independent determinations.
Figure 9
Selective stabilization of p58 that is p64-associated. (a) Rate of degradation of total GSTp58 (diamonds) compared to degradation of GSTp58 complexed with p64 (circles). The amount of total GSTp58 was determined by blotting as described in Fig. 8. The amount of GSTp58 in complex with p64 was determined by isolating GSTp58 on glutathione-Sepharose and then blotting for p64. (b) Western blot of the data shown in part a. (c) Degradation of total GSTp58 and p64-associated GSTp58 in cells overexpressing p64. (d) Model interpreting the data in a and c showing two pools of p58: an unstable pool of free p58 (circles) and a stable pool of p64-bound p58 (oval and circle).
Figure 10
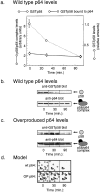
Models for individual CBF3 subunits and for the CBF3-CDEIII DNA complex. (a) Representation of CBF3 subunits as ellipsoids (with circular cross-sections) whose longest axis is equal to twice the Stokes radius and whose shorter axis is calculated using the experimentally determined mass and a typical value for protein density. p64 is shown as having two domains. Interactions that have been detected among CBF3 subunits are shown with heavy arrows. X denotes those interactions that have not been be detected in solution. (b) Enlarged cross-section of the CBF3-DNA complex showing the interaction of p58 and p64 with conserved bases in CDEIII. The boxes mark bases that are absolutely conserved among all 16 yeast centromeres and arrows indicate the positions of the putative p64 half-sites. Cross-linking data are summarized from Espelin et al. (1997). Below the enlargement is the proposed arrangement of CBF3 subunits on CDEIII DNA. A 184-bp piece of DNA with an extended CBF3 complex is shown. For a sense of scale, the dotted circles show the approximate diameter of a nucleosome and a microtubule in cross-section. The dotted box indicates the region of the model used to generate the cross-sectional view shown in section c. A model for organization of the core CBF3-CDEIII complex on 56 bp of centromeric DNA. The complex contains one p58 subunit, one p23Skp1, two p64s, and two p110s. The calculated mass of this complex (450 kD) and the experimentally determined sedimentation coefficient of 15 S yield a Stokes radius of 70 Å. CDEIII has been bent at an arbitrary position to conform to this 70 Å length. (d) Model for the regulation of CBF3 formation by p58. Newly synthesized p58 is activated by phosphorylation in a p23Skp1-dependent manner. Both active and inactive p58 are subject to ubiquitination and subsequent degradation. p58 bound by p64 assumes a conformational state in which it is resistant to degradation. However, p64-bound p58 remains in equilibrium with the free p58. Association of the p58/p64 complex with p110 and centromeric DNA leads to the formation of a stable complex that dissociates only very slowly.
Similar articles
- Probing the architecture of a simple kinetochore using DNA-protein crosslinking.
Espelin CW, Kaplan KB, Sorger PK. Espelin CW, et al. J Cell Biol. 1997 Dec 15;139(6):1383-96. doi: 10.1083/jcb.139.6.1383. J Cell Biol. 1997. PMID: 9396745 Free PMC article. - Interaction of yeast kinetochore proteins with centromere-protein/transcription factor Cbf1.
Hemmerich P, Stoyan T, Wieland G, Koch M, Lechner J, Diekmann S. Hemmerich P, et al. Proc Natl Acad Sci U S A. 2000 Nov 7;97(23):12583-8. doi: 10.1073/pnas.97.23.12583. Proc Natl Acad Sci U S A. 2000. PMID: 11070082 Free PMC article. - Structural basis for assembly of the CBF3 kinetochore complex.
Leber V, Nans A, Singleton MR. Leber V, et al. EMBO J. 2018 Jan 17;37(2):269-281. doi: 10.15252/embj.201798134. Epub 2017 Dec 6. EMBO J. 2018. PMID: 29212814 Free PMC article. - The Saccharomyces cerevisiae kinetochore.
Lechner J, Ortiz J. Lechner J, et al. FEBS Lett. 1996 Jun 24;389(1):70-4. doi: 10.1016/0014-5793(96)00563-7. FEBS Lett. 1996. PMID: 8682209 Review. - The role of centromere-binding factor 3 (CBF3) in spindle stability, cytokinesis, and kinetochore attachment.
Bouck D, Bloom K. Bouck D, et al. Biochem Cell Biol. 2005 Dec;83(6):696-702. doi: 10.1139/o05-161. Biochem Cell Biol. 2005. PMID: 16333320 Review.
Cited by
- The ubiquitin-proteasome system of Saccharomyces cerevisiae.
Finley D, Ulrich HD, Sommer T, Kaiser P. Finley D, et al. Genetics. 2012 Oct;192(2):319-60. doi: 10.1534/genetics.112.140467. Genetics. 2012. PMID: 23028185 Free PMC article. Review. - The kinetochore protein Ndc10p is required for spindle stability and cytokinesis in yeast.
Bouck DC, Bloom KS. Bouck DC, et al. Proc Natl Acad Sci U S A. 2005 Apr 12;102(15):5408-13. doi: 10.1073/pnas.0405925102. Epub 2005 Apr 4. Proc Natl Acad Sci U S A. 2005. PMID: 15809434 Free PMC article. - Saccharomyces cerevisiae BUB2 prevents mitotic exit in response to both spindle and kinetochore damage.
Krishnan R, Pangilinan F, Lee C, Spencer F. Krishnan R, et al. Genetics. 2000 Oct;156(2):489-500. doi: 10.1093/genetics/156.2.489. Genetics. 2000. PMID: 11014800 Free PMC article. - The abundance of Met30p limits SCF(Met30p) complex activity and is regulated by methionine availability.
Smothers DB, Kozubowski L, Dixon C, Goebl MG, Mathias N. Smothers DB, et al. Mol Cell Biol. 2000 Nov;20(21):7845-52. doi: 10.1128/MCB.20.21.7845-7852.2000. Mol Cell Biol. 2000. PMID: 11027256 Free PMC article. - Insights into Centromere DNA Bending Revealed by the Cryo-EM Structure of the Core Centromere Binding Factor 3 with Ndc10.
Zhang W, Lukoyanova N, Miah S, Lucas J, Vaughan CK. Zhang W, et al. Cell Rep. 2018 Jul 17;24(3):744-754. doi: 10.1016/j.celrep.2018.06.068. Cell Rep. 2018. PMID: 30021170 Free PMC article.
References
- Bai C, Sen P, Hofmann K, Ma L, Goebl M, Harper JW, Elledge SJ. SKP1connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell. 1996;86:263–274. - PubMed
- Clarke L. Centromeres: proteins, protein complexes, and repeated domains at centromeres of simple eukaryotes. Curr Opin Genet Dev. 1998;8:212–218. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous