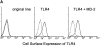MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4 - PubMed (original) (raw)
MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4
R Shimazu et al. J Exp Med. 1999.
Abstract
Toll-like receptor 4 (TLR4) is a mammalian homologue of Drosophila Toll, a leucine-rich repeat molecule that can trigger innate responses against pathogens. The TLR4 gene has recently been shown to be mutated in C3H/HeJ and C57BL/10ScCr mice, both of which are low responders to lipopolysaccharide (LPS). TLR4 may be a long-sought receptor for LPS. However, transfection of TLR4 does not confer LPS responsiveness on a recipient cell line, suggesting a requirement for an additional molecule. Here, we report that a novel molecule, MD-2, is requisite for LPS signaling of TLR4. MD-2 is physically associated with TLR4 on the cell surface and confers responsiveness to LPS. MD-2 is thus a link between TLR4 and LPS signaling. Identification of this new receptor complex has potential implications for understanding host defense, as well as pathophysiologic, mechanisms.
Figures
Figure 1
The nucleotide sequence of human MD-2 and its similarity to human MD-1. (A) The nucleotide sequence is shown with a deduced amino acid sequence. The signal peptide and canonical _N_-glycosylation sites are underlined. The stop codon is denoted by an asterisk. The nucleotide sequence of MD-2 will appear in the EMBL/GenBank/DDBJ nucleotide sequence databases under accession no. AB018549. (B) Amino acid sequences of human MD-1 and MD-2 were aligned using the BLAST2 program at the National Center for Biological Information (Bethesda, MD).
Figure 2
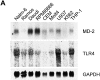
Expression of the MD-2 transcript. (A) Total RNA (20 μg/lane) from the indicated human cell lines was electrophoresed and hybridized with probes for human MD-2, TLR4, and GAPDH as indicated. Nalm-6, Ramos, Daudi, and RPMI8866 are of B lymphocyte lineage. CEM and Molt4 are of T cell origin. U937 and THP-1 are of monocytic origin. K562 is the erythroleukemia line. (B) Total RNA (20 μg/lane) from the indicated mouse tissues was used for Northern hybridization. The cDNA clone encoding mouse MD-2 was used as a probe. For GAPDH, the rat cDNA clone was used.
Figure 2
Expression of the MD-2 transcript. (A) Total RNA (20 μg/lane) from the indicated human cell lines was electrophoresed and hybridized with probes for human MD-2, TLR4, and GAPDH as indicated. Nalm-6, Ramos, Daudi, and RPMI8866 are of B lymphocyte lineage. CEM and Molt4 are of T cell origin. U937 and THP-1 are of monocytic origin. K562 is the erythroleukemia line. (B) Total RNA (20 μg/lane) from the indicated mouse tissues was used for Northern hybridization. The cDNA clone encoding mouse MD-2 was used as a probe. For GAPDH, the rat cDNA clone was used.
Figure 3
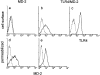
Membrane anchoring of MD-2 via TLR4. A stable line expressing MD-2 with the flag epitope was stained with the anti-flag mAb, followed by goat anti–mouse IgG–FITC (a and d). Staining with cell permeabilization is shown in d. Another stable transfectant was used that expressed MD-2 with the protein C epitope and TLR4 in b, c, and e. Cell surface MD-2 and TLR4 are shown with the rat anti–protein C mAb or the mouse anti–human TLR4 mAb HTA125 (b and c, respectively). MD-2 expression in permeabilized cells is shown in e. The second reagents used were goat anti–rat IgG–PE or goat anti–mouse IgG–FITC, respectively. Control histograms stained with isotype-matched mouse or rat mAb are shown (dotted lines).
Figure 4
MD-2 is coprecipitated with TLR4. (A) Stable transfectants expressing MD-2 alone or with TLR4 were stained with or without cell permeabilization. The intracellular MD-2 precursor was stained with the anti-protein C mAb. The specificity of the anti-TLR4 mAb was shown by cell surface staining of stable transfectants. Goat anti– mouse IgG–FITC was used as the second reagent. Dotted lines correspond to histograms stained with the second reagent alone. Panels correspond to the original Ba/F3 line, a transfectant expressing TLR4 alone, and a transfectant expressing TLR4 + MD-2, respectively. (B) Stable transfectants expressing TLR4 and MD-2 were subjected to immunoprecipitation with control mouse IgG (lane 1) or the anti-TLR4 mAb, HTA125 (lanes 2 and 3). After blotting, precipitated molecules were detected with an anti-flag mAb, M2. The precipitates shown in lanes 1 and 2 are from the transfectant expressing TLR4 and MD-2, both of which were tagged with the flag epitope. In lane 3, we used the control line in which the flag epitope was on TLR4 but not on MD-2.
Figure 4
MD-2 is coprecipitated with TLR4. (A) Stable transfectants expressing MD-2 alone or with TLR4 were stained with or without cell permeabilization. The intracellular MD-2 precursor was stained with the anti-protein C mAb. The specificity of the anti-TLR4 mAb was shown by cell surface staining of stable transfectants. Goat anti– mouse IgG–FITC was used as the second reagent. Dotted lines correspond to histograms stained with the second reagent alone. Panels correspond to the original Ba/F3 line, a transfectant expressing TLR4 alone, and a transfectant expressing TLR4 + MD-2, respectively. (B) Stable transfectants expressing TLR4 and MD-2 were subjected to immunoprecipitation with control mouse IgG (lane 1) or the anti-TLR4 mAb, HTA125 (lanes 2 and 3). After blotting, precipitated molecules were detected with an anti-flag mAb, M2. The precipitates shown in lanes 1 and 2 are from the transfectant expressing TLR4 and MD-2, both of which were tagged with the flag epitope. In lane 3, we used the control line in which the flag epitope was on TLR4 but not on MD-2.
Figure 5
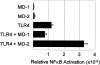
MD-2 enhances ligand-independent signaling via TLR4. A human kidney cell line, 293T, was transfected with expression vectors encoding the molecules indicated. A reporter plasmid for NF-κB activity and an expression vector encoding β-galactosidase was also transfected (see Materials and Methods). Relative NF-κB activity was calculated by normalizing luciferase activity with β-galactosidase activity. Data are shown as mean values from triplicate wells. Error bars, SD.
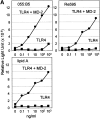
Figure 6
MD-2 confers LPS signaling on TLR4. (A) Stable transfectants (see Materials and Methods for details) expressing TLR4 alone (▪) or TLR4 and MD-2 (•) were stimulated with LPS from E. coli 055:B5, S. minnesota Re595, or lipid A at the concentrations indicated. After a 4-h culture, cells were harvested, and luciferase activity was determined and expressed as relative light units. (B) The transfectant expressing TLR4 and MD-2 was stimulated with LPS from E. coli 055: B5, LPS from S. minnesota Re595, or lipid A at 100 ng/ml. The mAb HTA125 (black bars) or a control mAb (gray bars) were included in the indicated groups (10 μg/ml). After 4 h, NF-κB activity was determined. Data represent mean values from triplicate wells.
Figure 6
MD-2 confers LPS signaling on TLR4. (A) Stable transfectants (see Materials and Methods for details) expressing TLR4 alone (▪) or TLR4 and MD-2 (•) were stimulated with LPS from E. coli 055:B5, S. minnesota Re595, or lipid A at the concentrations indicated. After a 4-h culture, cells were harvested, and luciferase activity was determined and expressed as relative light units. (B) The transfectant expressing TLR4 and MD-2 was stimulated with LPS from E. coli 055: B5, LPS from S. minnesota Re595, or lipid A at 100 ng/ml. The mAb HTA125 (black bars) or a control mAb (gray bars) were included in the indicated groups (10 μg/ml). After 4 h, NF-κB activity was determined. Data represent mean values from triplicate wells.
Similar articles
- Involvement of TLR4/MD-2 complex in species-specific lipopolysaccharide-mimetic signal transduction by Taxol.
Kawasaki K, Akashi S, Shimazu R, Yoshida T, Miyake K, Nishijima M. Kawasaki K, et al. J Endotoxin Res. 2001;7(3):232-6. J Endotoxin Res. 2001. PMID: 11581576 - Innate recognition of lipopolysaccharide by Toll-like receptor 4/MD-2 and RP105/MD-1.
Miyake K, Ogata H, Nagai Y, Akashi S, Kimoto M. Miyake K, et al. J Endotoxin Res. 2000;6(5):389-91. J Endotoxin Res. 2000. PMID: 11521060 - Positional cloning of Lps, and the general role of toll-like receptors in the innate immune response.
Beutler B, Poltorak A. Beutler B, et al. Eur Cytokine Netw. 2000 Jun;11(2):143-52. Eur Cytokine Netw. 2000. PMID: 10903793 Review. - Role of MD-2 in TLR2- and TLR4-mediated recognition of Gram-negative and Gram-positive bacteria and activation of chemokine genes.
Dziarski R, Gupta D. Dziarski R, et al. J Endotoxin Res. 2000;6(5):401-5. doi: 10.1179/096805100101532243. J Endotoxin Res. 2000. PMID: 11521063 Review.
Cited by
- Irradiated whole cell Chlamydia vaccine confers significant protection in a murine genital tract challenge model.
Broder KC, Matrosova VY, Tkavc R, Gaidamakova EK, Ho LTVT, Macintyre AN, Soc A, Diallo A, Darnell SC, Bash S, Daly MJ, Jerse AE, Liechti GW. Broder KC, et al. NPJ Vaccines. 2024 Nov 11;9(1):207. doi: 10.1038/s41541-024-00968-z. NPJ Vaccines. 2024. PMID: 39528548 Free PMC article. - Protective role of aconitate decarboxylase 1 in neuroinflammation-induced dysfunctions of the paraventricular thalamus and sleepiness.
Chang J, Li Z, Yuan H, Wang X, Xu J, Yang P, Qin L. Chang J, et al. Commun Biol. 2024 Nov 10;7(1):1484. doi: 10.1038/s42003-024-07215-0. Commun Biol. 2024. PMID: 39523388 Free PMC article. - Biosynthetic enzyme analysis identifies a protective role for TLR4-acting gut microbial sulfonolipids in inflammatory bowel disease.
Older EA, Zhang J, Ferris ZE, Xue D, Zhong Z, Mitchell MK, Madden M, Wang Y, Chen H, Nagarkatti P, Nagarkatti M, Fan D, Ellermann M, Li YX, Li J. Older EA, et al. Nat Commun. 2024 Oct 30;15(1):9371. doi: 10.1038/s41467-024-53670-y. Nat Commun. 2024. PMID: 39477928 Free PMC article. - A myeloid differentiation-like protein in partnership with Toll5 from the pest insect Spodoptera litura senses baculovirus infection.
Zhang R, Zhong J, Li Y, Li M, Zhang J, Hu Q, Wen L, Xu X, Jin F, Yang W, Lu Y, Strand MR, Yu XQ. Zhang R, et al. Proc Natl Acad Sci U S A. 2024 Oct 29;121(44):e2415398121. doi: 10.1073/pnas.2415398121. Epub 2024 Oct 23. Proc Natl Acad Sci U S A. 2024. PMID: 39441638 - Amine headgroups in ionizable lipids drive immune responses to lipid nanoparticles by binding to the receptors TLR4 and CD1d.
Chaudhary N, Kasiewicz LN, Newby AN, Arral ML, Yerneni SS, Melamed JR, LoPresti ST, Fein KC, Strelkova Petersen DM, Kumar S, Purwar R, Whitehead KA. Chaudhary N, et al. Nat Biomed Eng. 2024 Nov;8(11):1483-1498. doi: 10.1038/s41551-024-01256-w. Epub 2024 Oct 3. Nat Biomed Eng. 2024. PMID: 39363106
References
- Hammond-Kosak KE, Jones JDG. Plant disease resistance genes. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:575–607. - PubMed
- Lemaitre B, Nicolas E, Michaut L, Reichhart J-M, Hoffmann JA. The dorsoventral regulatory gene cassette spaetzle/Toll/cactus controls the potent antifungal response in Drosophilaadults. Cell. 1996;86:973–983. - PubMed
- Medzhitov R, Janeway CA., Jr Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997;9:4–9. - PubMed
- Vogel G. Fly development genes lead to immune find. Science. 1998;281:1942–1944. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous