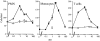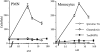Thioredoxin, a redox enzyme released in infection and inflammation, is a unique chemoattractant for neutrophils, monocytes, and T cells - PubMed (original) (raw)
. 1999 Jun 7;189(11):1783-9.
doi: 10.1084/jem.189.11.1783.
O M Howard, H F Dong, J J Oppenheim, C Bizzarri, R Sergi, G Caselli, S Pagliei, B Romines, J A Wilshire, M Mengozzi, H Nakamura, J Yodoi, K Pekkari, R Gurunath, A Holmgren, L A Herzenberg, L A Herzenberg, P Ghezzi
Affiliations
- PMID: 10359582
- PMCID: PMC2193090
- DOI: 10.1084/jem.189.11.1783
Thioredoxin, a redox enzyme released in infection and inflammation, is a unique chemoattractant for neutrophils, monocytes, and T cells
R Bertini et al. J Exp Med. 1999.
Abstract
Thioredoxin (Trx) is a ubiquitous intracellular protein disulfide oxidoreductase with a CXXC active site that can be released by various cell types upon activation. We show here that Trx is chemotactic for monocytes, polymorphonuclear leukocytes, and T lymphocytes, both in vitro in the standard micro Boyden chamber migration assay and in vivo in the mouse air pouch model. The potency of the chemotactic action of Trx for all leukocyte populations is in the nanomolar range, comparable with that of known chemokines. However, Trx does not increase intracellular Ca2+ and its activity is not inhibited by pertussis toxin. Thus, the chemotactic action of Trx differs from that of known chemokines in that it is G protein independent. Mutation of the active site cysteines resulted in loss of chemotactic activity, suggesting that the latter is mediated by the enzyme activity of Trx. Trx also accounted for part of the chemotactic activity released by human T lymphotropic virus (HTLV)-1-infected cells, which was inhibited by incubation with anti-Trx antibody. Since Trx production is induced by oxidants, it represents a link between oxidative stress and inflammation that is of particular interest because circulating Trx levels are elevated in inflammatory diseases and HIV infection.
Figures
Figure 6
Trx-induced leukocyte recruitment in the air pouch in mice. 1 μg of Trx was injected in the air pouch as described in Materials and Methods. Control mice were injected with vehicle only. 4 h later, the cells in the air pouch were recovered, counted, and analyzed by FACS®. ○, mean of seven control mice; •, Trx-treated mice (n = 7).
Figure 1
Chemotactic effect of Trx on human PMNs, monocytes, and T cells. The average number of cells migrated in five oil immersion fields ± SD using different concentrations of human recombinant Trx (•) or boiled (30 min at 100°C) human recombinant Trx (○) are shown. The response to a known chemokine (left panel, IL-8; center panel, MCP-1; right panel, RANTES) is also shown (⋄) as a reference.
Figure 2
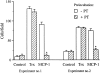
Chemotactic activity of Trx is inhibited by an anti-Trx antibody. Trx was incubated for 15 min at 37°C with 0.5 μg/ml goat anti– human Trx or a control antibody (anti-GST). Trx was then assayed for chemotactic activity for monocytes at the concentration of 2.5 nM, as described above. Data are mean ± SD of triplicate samples.
Figure 3
Chemotactic activity of Spirulina TRX, Trx(SGPS) mutant, or glutaredoxin on human PMNs and monocytes. The proteins were tested at the indicated concentrations, as described in the legend to Fig. 1. The data show the number of cells migrated in five oil immersion fields using different concentrations of the test protein. Data are mean ± SD of triplicate samples.
Figure 4
Trx does not increase cytosolic Ca2+ in human monocytes or PMNs. Traces represent levels of [Ca2+]i in single adherent monocytes (A and B) or PMNs (C and D) in response to 30 ng/ml (2.5 nM) Trx (A and C), 25 ng/ml (3 nM) IL-8 (D), or 50 ng/ml (6 nM) MCP-1 (B). Traces are representative of 12–32 cell recordings (7 donors, 3–5 cells per coverslip). Arrows indicate the addition of agents to the bathing medium, which causes a spike in the trace due to exposure to light.
Figure 5
Chemotactic activity of Trx on monocytes is not inhibited by PT. Monocytes were incubated with 1 μg/ml PT for 90 min at 37°C, then washed to remove PT and used for chemotaxis experiments with Trx (30 ng/ml) or MCP-1 (25 ng/ml), as described above. Data from two independent experiments are shown. Data are mean ± SD of triplicate samples. *P < 0.05 versus control by Student's t test.
Figure 7
Trx is a component of the chemotactic activity for PMNs released by the HTLV-1–transformed cell line MT4. Supernatants from MT4 cells or control media were treated for 15 min at 37°C with goat anti–human Trx (0.5 μg/ml) or a control antibody (anti-GST). Supernatants were then assayed for chemotactic activity on PMNs as described above. **P < 0.05 versus control by Student's t test.
Similar articles
- Circulating thioredoxin suppresses lipopolysaccharide-induced neutrophil chemotaxis.
Nakamura H, Herzenberg LA, Bai J, Araya S, Kondo N, Nishinaka Y, Herzenberg LA, Yodoi J. Nakamura H, et al. Proc Natl Acad Sci U S A. 2001 Dec 18;98(26):15143-8. doi: 10.1073/pnas.191498798. Epub 2001 Dec 11. Proc Natl Acad Sci U S A. 2001. PMID: 11742067 Free PMC article. - Requirements for the different cysteines in the chemotactic and desensitizing activity of human thioredoxin.
Bizzarri C, Holmgren A, Pekkari K, Chang G, Colotta F, Ghezzi P, Bertini R. Bizzarri C, et al. Antioxid Redox Signal. 2005 Sep-Oct;7(9-10):1189-94. doi: 10.1089/ars.2005.7.1189. Antioxid Redox Signal. 2005. PMID: 16115022 - Thioredoxin specifically cross-desensitizes monocytes to MCP-1.
Pagliei S, Ghezzi P, Bizzarri C, Sabbatini V, Frascaroli G, Sozzani S, Caselli G, Bertini R. Pagliei S, et al. Eur Cytokine Netw. 2002 Apr-Jun;13(2):261-7. Eur Cytokine Netw. 2002. PMID: 12101083 - Extracellular thioredoxin and thioredoxin-binding protein 2 in control of cancer.
Nakamura H, Masutani H, Yodoi J. Nakamura H, et al. Semin Cancer Biol. 2006 Dec;16(6):444-51. doi: 10.1016/j.semcancer.2006.09.001. Epub 2006 Sep 26. Semin Cancer Biol. 2006. PMID: 17095246 Review. - Redox regulation by thioredoxin superfamily; protection against oxidative stress and aging.
Tanaka T, Nakamura H, Nishiyama A, Hosoi F, Masutani H, Wada H, Yodoi J. Tanaka T, et al. Free Radic Res. 2000 Dec;33(6):851-5. doi: 10.1080/10715760000301361. Free Radic Res. 2000. PMID: 11237106 Review.
Cited by
- The role of thioredoxin 1 in the mycophenolic acid-induced apoptosis of insulin-producing cells.
Huh KH, Cho Y, Kim BS, Do JH, Park YJ, Joo DJ, Kim MS, Kim YS. Huh KH, et al. Cell Death Dis. 2013 Jul 11;4(7):e721. doi: 10.1038/cddis.2013.247. Cell Death Dis. 2013. PMID: 23846223 Free PMC article. - Multi-cohort analysis of host immune response identifies conserved protective and detrimental modules associated with severity across viruses.
Zheng H, Rao AM, Dermadi D, Toh J, Murphy Jones L, Donato M, Liu Y, Su Y, Dai CL, Kornilov SA, Karagiannis M, Marantos T, Hasin-Brumshtein Y, He YD, Giamarellos-Bourboulis EJ, Heath JR, Khatri P. Zheng H, et al. Immunity. 2021 Apr 13;54(4):753-768.e5. doi: 10.1016/j.immuni.2021.03.002. Epub 2021 Mar 24. Immunity. 2021. PMID: 33765435 Free PMC article. - Circulating thioredoxin suppresses lipopolysaccharide-induced neutrophil chemotaxis.
Nakamura H, Herzenberg LA, Bai J, Araya S, Kondo N, Nishinaka Y, Herzenberg LA, Yodoi J. Nakamura H, et al. Proc Natl Acad Sci U S A. 2001 Dec 18;98(26):15143-8. doi: 10.1073/pnas.191498798. Epub 2001 Dec 11. Proc Natl Acad Sci U S A. 2001. PMID: 11742067 Free PMC article. - Thioredoxin peroxidase secreted by Fasciola hepatica induces the alternative activation of macrophages.
Donnelly S, O'Neill SM, Sekiya M, Mulcahy G, Dalton JP. Donnelly S, et al. Infect Immun. 2005 Jan;73(1):166-73. doi: 10.1128/IAI.73.1.166-173.2005. Infect Immun. 2005. PMID: 15618151 Free PMC article. - Thioredoxin 80-activated-monocytes (TAMs) inhibit the replication of intracellular pathogens.
Cortes-Bratti X, Bassères E, Herrera-Rodriguez F, Botero-Kleiven S, Coppotelli G, Andersen JB, Masucci MG, Holmgren A, Chaves-Olarte E, Frisan T, Avila-Cariño J. Cortes-Bratti X, et al. PLoS One. 2011 Feb 18;6(2):e16960. doi: 10.1371/journal.pone.0016960. PLoS One. 2011. PMID: 21365006 Free PMC article.
References
- Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. - PubMed
- Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989;264:13963–13966. - PubMed
- Holmgren A, Bjornstedt M. Thioredoxin and thioredoxin reductase. Methods Enzymol. 1995;252:199–208. - PubMed
- Nakamura, H., M. Matsuda, K. Furuke, Y. Kitaoka, S. Iwata, K. Toda, T. Inamoto, Y. Yamaoka, K. Ozawa, and J. Yo. 1994. Adult T cell leukemia-derived factor/human thioredoxin protects endothelial F-2 cell injury caused by activated neutrophils or hydrogen peroxide. Immunol. Lett. 42:75–80. (See published erratum 42:213.) - PubMed
- Sasada T, Iwata S, Sato N, Kitaoka Y, Hirota K, Nakamura K, Nishiyama A, Taniguchi Y, Takabayashi A, Yodoi J. Redox control of resistance to cis-diamminedichloroplatinum (II) (CDDP): protective effect of human thioredoxin against CDDP-induced cytotoxicity. J Clin Invest. 1996;97:2268–2276. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous