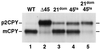The phosphatidylinositol 3-phosphate binding protein Vac1p interacts with a Rab GTPase and a Sec1p homologue to facilitate vesicle-mediated vacuolar protein sorting - PubMed (original) (raw)
The phosphatidylinositol 3-phosphate binding protein Vac1p interacts with a Rab GTPase and a Sec1p homologue to facilitate vesicle-mediated vacuolar protein sorting
G G Tall et al. Mol Biol Cell. 1999 Jun.
Free PMC article
Abstract
Activated GTP-bound Rab proteins are thought to interact with effectors to elicit vesicle targeting and fusion events. Vesicle-associated v-SNARE and target membrane t-SNARE proteins are also involved in vesicular transport. Little is known about the functional relationship between Rabs and SNARE protein complexes. We have constructed an activated allele of VPS21, a yeast Rab protein involved in vacuolar protein sorting, and demonstrated an allele-specific interaction between Vps21p and Vac1p. Vac1p was found to bind the Sec1p homologue Vps45p. Although no association between Vps21p and Vps45p was seen, a genetic interaction between VPS21 and VPS45 was observed. Vac1p contains a zinc-binding FYVE finger that may bind phosphatidylinositol 3-phosphate [PtdIns(3)P]. In other FYVE domain proteins, this motif and PtdIns(3)P are necessary for membrane association. Vac1 proteins with mutant FYVE fingers still associated with membranes but showed vacuolar protein sorting defects and reduced interactions with Vps45p and activated Vps21p. Vac1p membrane association was not dependent on PtdIns(3)P, Pep12p, Vps21p, Vps45p, or the PtdIns 3-kinase, Vps34p. Vac1p FYVE finger mutant missorting phenotypes were suppressed by a defective allele of VPS34. These data indicate that PtdIns(3)P may perform a regulatory role, possibly involved in mediating Vac1p protein-protein interactions. We propose that activated-Vps21p interacts with its effector, Vac1p, which interacts with Vps45p to regulate the Golgi to endosome SNARE complex.
Figures
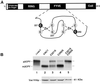
Figure 1
Purification and characterization of GTP binding and GTPase activities of Vps21p and Q66L-Vps21p. (A) His6-Vps21p and His6-Q66L-Vps21p were expressed in E. coli and purified using Ni-agarose as described in MATERIALS AND METHODS. One microgram of purified Vps21p (lane 1) and purified Q66L-Vps21p (lane 2) were resolved by SDS-PAGE and stained with Coomassie brilliant blue to visualize proteins. (B) His6-Vps21p and His6-Q66L-Vps21p (5.2 pmol each) were incubated in a GTP-binding reaction containing 0.025 mM [γ-32P]GTP (5000 Ci/ml) and 50 mM GTP for 15 min at 30°C. Aliquots from the binding reactions were passed through nitrocellulose filters, washed, dried, and the amount of protein-associated [γ-32P]GTP was determined by scintillation counting. (C) His6-Vps21p and His6-Q66L-Vps21p (2.96 pmol each) were prebound with [γ-32P]GTP and then incubated at 30°C in GTPase reactions as described in MATERIALS AND METHODS. At the specific time points indicated, an aliquot was removed from each reaction (equivalent to 329 fmol of Vps21p) and quenched by the addition of charcoal suspension. The charcoal and bound Vps21p, Vps21p-GTP, and free GTP were pelleted, and the amount of hydrolyzed γ-32P that remained in the supernatant was determined by liquid scintillation counting. No GTPase activity was seen using a BSA control (our unpublished data).
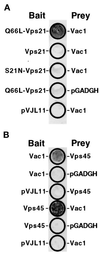
Figure 2
Vac1p interacts with Q66L-Vps21p and Vps45p in the yeast two-hybrid system. (A) The following L40 yeast cotransformants were patched onto a YNB-glucose plate lacking tryptophan and leucine: pGT21-3 (Q66L-Vps21 bait) and pGT1-9 (Vac1 prey), pGT21-1 (Vps21 bait) and pGT1-9, pGT21-2 (S21N-Vps21 bait) and pGT1-9, pGT21-3 and pGADGH, and pVJL11 and pGT1-9. After 3 d of growth at 30°C, these yeast were subjected to the β-galactosidase filter activity assay (Hama et al., 1999). Detectable β-galactosidase activity indicates a positive interaction between bait and prey proteins. (B) The following L40 yeast transformants were subjected to the β-galactosidase filter activity assay in the same manner as in A: pGT1-8 (Vac1 bait) and pGT45-2 (Vps45 prey), pGT1-8 and pGADGH, pVJL11 and pGT45-2, pGT45-1 (Vps45 bait) and pGT1-9 (Vac1 prey), pGT45-1 and pGADGH, and pVJL11 and pGT1-9.
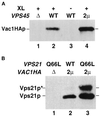
Figure 3
Vac1p interacts with Vps45p and Vps21p in vivo. (A) Spheroplasts (5 OD600 equivalents) of the following strains were prepared: GTY107 (Δvps45Δvac1) expressing Vac1HAp (lane 1), GTY104 (Δvac1) expressing Vac1HAp (lanes 2 and 3), and GTY104 expressing Vac1HAp and overexpressing Vps45p (lane 4). The spheroplasts were lysed, and the lysates were treated with (+) or without (−) the reducible cross-linker DSP (XL) as described. Vps45p and cross-linked proteins were immunoprecipitated with Vps45p antibodies. The immunoprecipitates were resolved by SDS-PAGE and transferred to nitrocellulose, and the blots were probed with HA antiserum. Vac1HAp was visualized using Blaze chemilluminescent detection reagents. (B) Spheroplasts of GTY112 (Δvac1Δvps21) (25 OD600 equivalents) overexpressing Q66L-Vps21p (lane 1), Vps21p and Vac1HAp (lane 2), or Q66L-Vps21p and Vac1HAp (lane 3) were lysed and centrifuged at 16,000 × g. HA antisera (10 μl) was added to the cleared supernatants and incubated for 30 min at 4°C. Protein G-Sepharose was added, and the reactions were incubated an additional 30 min at 4°C. Sepharose–antibody–antigen complexes were pelleted at 2000 × g and washed five times with native lysis buffer. Proteins were eluted with sample buffer, resolved by SDS-PAGE, and subjected to Western analysis using Vps21p antisera. Prenylated (22 kDa, Vps21p) and unprenylated (23 kDa, Vps21p*) Vps21p were detected using Blaze chemilluminescent detection reagents.
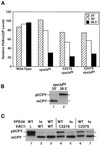
Figure 4
_vps45_ts and _VPS21_dom display a synthetic CPY missorting phenotype. SEY6210 (WT, lane 1), CCY45-2 (Δ45, lane 2), SEY6210 expressing pBHY21-49 (21dom, lane 3), CCY45Δ2 expressing pBHY45-7 (45ts, lane 4), and CCY45Δ2 expressing pBHY21-49 and pBHY45-7 (21dom/45ts, lane 5) were pulse labeled with [35S]ProMix for 10 min at 28.5°C in YNB-glucose containing 25 mM KH2PO4, pH 5.4. Chase solution with unlabeled methionine and cysteine was added, and the cells were incubated for an additional 30 min before being quenched with TCA. CPY was then immunoprecipitated from the combined intracellular and extracellular fractions of these cells and resolved by SDS-PAGE. The positions of mature vacuolar (m) and the Golgi-modified forms (p2) of CPY are denoted.
Figure 5
Characterization of vac1 FYVE finger mutations. (A) Vac1p consists of the following previously identified domains: a C2H2 TFIIIA-like zinc finger located proximal to the N terminus, an H6 variant zinc-binding RING finger, a zinc-binding FYVE RING finger, and a region predicted to form coiled coil structures located at the C terminus (Burd et al., 1997). Shown below the FYVE finger is an enlargement of the predicted structure of this domain along with the highlighted cysteine residues that have been mutated to serines individually or in combination (C221S, C237S, and C292S). (B) The abilities of yeast strains expressing vac1 alleles encoding Vac1 proteins with mutant FYVE domains to sort CPY were assessed. GTY104 (SEY6210; vac1Δ3:: NEO; lane 1) was transformed with plasmids pGT1-1 (WT VAC1) (lane 2), pGT1-4 (C221S-vac1) (lane 3), pGT1-6 (C292S-vac1) (lane 4), and pGT1-7 (C221/292S-vac1) (lane 5). CPY was immunoprecipitated from the labeled lysates that were generated from these strains as described in MATERIALS AND METHODS and in Figure 4 but at 30°C (upper panel). The expression levels of C-terminal HA-tagged Vac1p FYVE finger point mutants were also examined under steady-state conditions. GTY104 (1.0 OD600 equivalent) expressing the following HA-tagged vac1 alleles were lysed in urea sample buffer: pGT1-17 (WT Vac1HA) (lane 2), pGT1-18 (C221S-Vac1HA) (lane 3), pGT1-19 (C292S-Vac1HA) (lane 4), and pGT1-20 (C221SC292S-Vac1HA) (lane 5). The lysates were resolved by SDS-PAGE, and the Vac1HA proteins were identified by Western blot analysis using HA antibodies. Vac1HAp was detected using the Blaze chemilluminescent detection system (lower panel).
Figure 6
The membrane association of Vac1HAp is not dependent on the functions of Pep12p, Vps34p, Vps21p, Vps45p, or the Vac1p FYVE finger. The subcellular fractionation patterns of Vac1HAp were assessed in the following strains that expressed Vac1HAp from a CEN plasmid; GTY104 (WT, panels 1 and 2), GTY108 (Δpep12, panel 3), GTY109 (Δvps34, panel 4), GTY111 (Δpep12Δvps34, panel 5), GTY106, (Δvps21, panel 6), and GTY107 (Δvps45, panel 7) and that expressed C221/292S-Vac1HAp from a CEN plasmid, GTY104 (C221/292S-Vac1HAp, panel 8). Spheroplasts (20 OD600 equivalents) of these strains were lysed, and unbroken cells were cleared by centrifugation at 500 × g. Five OD600 equivalents of these supernatants were then centrifuged at 13,000 × g to generate a P13 pellet and an S13 supernatant. Five OD600 equivalents of the S13 were further fractionated by spinning at 100,000 × g to generate a P100 pellet and an S100 supernatant. Supernatants were precipitated with TCA and resuspended in urea sample buffer as described. Pellets were directly resuspended in urea sample buffer. All fractions were resolved by SDS-PAGE, and Vac1HA proteins were identified by Western blot analysis using HA antibodies. Vac1HAp and C221/292S-Vac1HAp were visualized using Blaze chemilluminescent detection reagents.
Figure 7
A _vps34_ts mutant suppresses the partial missorting phenotypes of vac1 FYVE finger mutants. (A) DDY3477 (_vps34_ts) displays temperature-sensitive PtdIns(3) kinase activity. Wild-type yeast and DDY3477 yeast expressing wild-type VAC1, C221S-vac1, or C237S-vac1 were grown for 16 h at 25 or 30°C in minimal media containing [3H]_myo_-inositol. A 0.1 vol of YPD was added, and incubations were continued for 2.5 h. One 30°C culture was shifted to 38.5°C for the last 30 min of the 2.5-h incubation. Deacylated lipid extracts were prepared from lysates of these cultures and separated by HPLC. This procedure allowed the complete separation of the deacylated glycerophosphoinositols gPI(3)P and gPI(4)P. For each strain, the amount of gPI(3)P that was produced is expressed relative to the amount of gPI(4)P, whose level remained largely unchanged at 25, 30, and 38.5°C. (B) DDY3477 (_vps34_ts) displayed a temperature-sensitive vacuolar protein sorting defect. The ability of the _vps34_ts strain DDY3477 to sort CPY was determined as described in Figure 4 and MATERIALS AND METHODS, except that cultures were incubated at 25 or 38.5°C for 10 min before labeling at those temperatures. (C) The abilities of vac1 FYVE finger mutants to sort CPY to the vacuole were compared in wild-type and _vps34_ts backgrounds at the semipermissive temperature of 30°C. GTY104 (Δvac1) expressing no Vac1p (lane 1), VAC1 (lane 3), C221S-vac1 (lane 4), and C237S-vac1 (lane 6) and GTY110 (_Δvac1vps34_ts) expressing VAC1 (lane 2), C221S-vac1 (lane 5), and C237S-vac1 (lane 7) were pulse labeled with [35S]ProMix for 10 min and chased with unlabeled amino acids for 30 min at 30°C. CPY was immunoprecipitated from the combined intracellular and extracellular fractions of lysates generated from these cells as described in Figure 4 and MATERIALS AND METHODS.
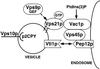
Figure 8
Model of yeast Golgi-to-endosome vesicle-mediated protein transport. Golgi-modified p2 CPY is bound by the sorting receptor Vps10p and packaged into transport vesicles that bud from the Golgi. The v-SNARE Vti1p and the GTPase Vps21p that has been activated by the Vps21p exchange factor Vps9p are present on these vesicles as they move through the cytoplasm to the prevacuolar endosomal compartment. Vac1p then interacts with activated GTP-bound Vps21p on the vesicle and also with Vps45p that is peripherally associated with the endosome via the t-SNARE homologue Pep12p. These interactions may be regulated by PtdIns(3)P. The signal transduced from activated Vps21p through Vac1p and Vps45p enables the t-SNARE homologue Pep12p to interact with Vti1p to promote vesicle fusion.
Similar articles
- A novel Sec18p/NSF-dependent complex required for Golgi-to-endosome transport in yeast.
Burd CG, Peterson M, Cowles CR, Emr SD. Burd CG, et al. Mol Biol Cell. 1997 Jun;8(6):1089-104. doi: 10.1091/mbc.8.6.1089. Mol Biol Cell. 1997. PMID: 9201718 Free PMC article. - Vac1p coordinates Rab and phosphatidylinositol 3-kinase signaling in Vps45p-dependent vesicle docking/fusion at the endosome.
Peterson MR, Burd CG, Emr SD. Peterson MR, et al. Curr Biol. 1999 Feb 11;9(3):159-62. doi: 10.1016/s0960-9822(99)80071-2. Curr Biol. 1999. PMID: 10021387 - Vps9p is a guanine nucleotide exchange factor involved in vesicle-mediated vacuolar protein transport.
Hama H, Tall GG, Horazdovsky BF. Hama H, et al. J Biol Chem. 1999 May 21;274(21):15284-91. doi: 10.1074/jbc.274.21.15284. J Biol Chem. 1999. PMID: 10329739 - Receptor-mediated protein sorting to the vacuole in yeast: roles for a protein kinase, a lipid kinase and GTP-binding proteins.
Stack JH, Horazdovsky B, Emr SD. Stack JH, et al. Annu Rev Cell Dev Biol. 1995;11:1-33. doi: 10.1146/annurev.cb.11.110195.000245. Annu Rev Cell Dev Biol. 1995. PMID: 8689553 Review. - Novel pathways, membrane coats and PI kinase regulation in yeast lysosomal trafficking.
Burd CG, Babst M, Emr SD. Burd CG, et al. Semin Cell Dev Biol. 1998 Oct;9(5):527-33. doi: 10.1006/scdb.1998.0255. Semin Cell Dev Biol. 1998. PMID: 9835640 Review.
Cited by
- Role of Rabenosyn-5 and Rab5b in host cell cytosol uptake reveals conservation of endosomal transport in malaria parasites.
Sabitzki R, Roßmann AL, Schmitt M, Flemming S, Guillén-Samander A, Behrens HM, Jonscher E, Höhn K, Fröhlke U, Spielmann T. Sabitzki R, et al. PLoS Biol. 2024 May 31;22(5):e3002639. doi: 10.1371/journal.pbio.3002639. eCollection 2024 May. PLoS Biol. 2024. PMID: 38820535 Free PMC article. - SNARE chaperone Sly1 directly mediates close-range vesicle tethering.
Duan M, Plemel RL, Takenaka T, Lin A, Delgado BM, Nattermann U, Nickerson DP, Mima J, Miller EA, Merz AJ. Duan M, et al. J Cell Biol. 2024 Jun 3;223(6):e202001032. doi: 10.1083/jcb.202001032. Epub 2024 Mar 13. J Cell Biol. 2024. PMID: 38478018 Free PMC article. - Characterization of the Expression of Vacuolar Protein Sorting 11 (Vps11) in Mammalian Oligodendrocytes.
Skoff RP, Bessert D, Banerjee S, Luo X, Thummel R. Skoff RP, et al. ASN Neuro. 2021 Jan-Dec;13:17590914211009851. doi: 10.1177/17590914211009851. ASN Neuro. 2021. PMID: 33874780 Free PMC article. - Rab5 GTPases are required for optimal TORC2 function.
Locke MN, Thorner J. Locke MN, et al. J Cell Biol. 2019 Mar 4;218(3):961-976. doi: 10.1083/jcb.201807154. Epub 2018 Dec 21. J Cell Biol. 2019. PMID: 30578283 Free PMC article. - The Na+(K+)/H+ exchanger Nhx1 controls multivesicular body-vacuolar lysosome fusion.
Karim MA, Brett CL. Karim MA, et al. Mol Biol Cell. 2018 Feb 1;29(3):317-325. doi: 10.1091/mbc.E17-08-0496. Epub 2017 Dec 6. Mol Biol Cell. 2018. PMID: 29212874 Free PMC article.
References
- Aalto MK, Ruohonen L, Hosono K, Keranen S. Cloning and sequencing of the yeast Saccharomyces cerevisiae SEC1 gene localized on chromosome IV. Yeast. 1991;7:643–650. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases