Microtubule-based endoplasmic reticulum motility in Xenopus laevis: activation of membrane-associated kinesin during development - PubMed (original) (raw)
Microtubule-based endoplasmic reticulum motility in Xenopus laevis: activation of membrane-associated kinesin during development
J D Lane et al. Mol Biol Cell. 1999 Jun.
Free PMC article
Abstract
The endoplasmic reticulum (ER) in animal cells uses microtubule motor proteins to adopt and maintain its extended, reticular organization. Although the orientation of microtubules in many somatic cell types predicts that the ER should move toward microtubule plus ends, motor-dependent ER motility reconstituted in extracts of Xenopus laevis eggs is exclusively a minus end-directed, cytoplasmic dynein-driven process. We have used Xenopus egg, embryo, and somatic Xenopus tissue culture cell (XTC) extracts to study ER motility during embryonic development in Xenopus by video-enhanced differential interference contrast microscopy. Our results demonstrate that cytoplasmic dynein is the sole motor for microtubule-based ER motility throughout the early stages of development (up to at least the fifth embryonic interphase). When egg-derived ER membranes were incubated in somatic XTC cytosol, however, ER tubules moved in both directions along microtubules. Data from directionality assays suggest that plus end-directed ER tubule extensions contribute approximately 19% of the total microtubule-based ER motility under these conditions. In XTC extracts, the rate of ER tubule extensions toward microtubule plus ends is lower ( approximately 0.4 microm/s) than minus end-directed motility ( approximately 1.3 microm/s), and plus end-directed motility is eliminated by a function-blocking anti-conventional kinesin heavy chain antibody (SUK4). In addition, we provide evidence that the initiation of plus end-directed ER motility in somatic cytosol is likely to occur via activation of membrane-associated kinesin.
Figures
Figure 1
Kinesin-coated bead motility for the analysis of ER tubule directionality. Membrane motility is recorded on videotape (A), and the membrane network is permeabilized and washed away while microtubule networks are stabilized by flowing through acetate buffer containing 0.1% Triton X-100 and 20 μM Taxol (B). Subsequently, carboxylated beads, precoated with pig brain kinesin, are flowed in, and the orientation of microtubules within the field is determined by observing the direction of kinesin-coated bead motility (C). Bar, 0.5 μm.
Figure 2
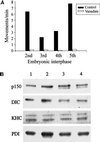
Analysis of membrane-associated motors and the sensitivity of ER movement to vanadate in extracts prepared from the first five embryonic interphases. (A) Sodium orthovanadate (20 μM) abolishes ER motility in Xenopus embryo extracts prepared from cycling extracts (second interphase) and in vitro fertilized, freshly laid eggs (third, fourth, and fifth interphases). Membrane movement was quantitated by counting the numbers of tubule extensions in 10 randomly selected microscope fields, each observed for 2 min, in the presence or absence of vanadate (average of at least two assays). (B) Analysis of ER-associated motors during Xenopus embryonic development. Floated membranes obtained from egg and embryo extracts were analyzed by immunoblotting using antibodies against p150Glued (p150), Xenopus dynein intermediate chain (DIC), KHC, and PDI (loading control). Floated membranes from eggs and embryos are shown during first interphase (lane 1), third interphase (lane 2), fourth interphase (lane 3), and fifth interphase (lane 4).
Figure 3
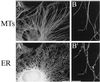
Immunofluorescence images of fixed Xenopus XTC cells labeled with antibodies against microtubules (MTs, top) and the ER (bottom). (A and A′) Portion of a typical interphase XTC cell showing the extended, reticular morphology of the ER. Bar, 10 μm (B and B′) Confocal images of the periphery of an XTC cell showing the close association between microtubules and the ER (in most cases, microtubules and ER tubules do not coalign exactly; instead, the ER tubules appear to be associated with microtubules at discrete sites along their lengths, as well as at their tips). Bar, = 5 μm. Microtubules and the ER were visualized using a rat monoclonal antibody raised against α-tubulin (clone YL1/2) and a mouse monoclonal antibody recognizing PDI (clone 1D3), respectively, and the appropriate anti-rat and anti-mouse fluorescently labeled secondary antibodies.
Figure 4
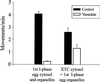
ER motility is only partially inhibited by vanadate in XTC cytosol. Floated egg ER was incubated in first interphase egg cytosol or in XTC cytosol, and ER motility was quantitated by counting the numbers of membrane tubule extensions in 10 randomly selected microscope fields, each observed for 2 min, in the presence or absence of 20 μM vanadate (average of at least three assays; bars indicate SEs).
Figure 5
An example of plus and minus end-directed motility of floated egg ER in XTC cytosol observed by VE-DIC. ER tubules are shown extending outward from the same body of membrane, in opposite directions along the same microtubule. A bead motility assay was also performed on this field of microtubules to determine the polarity of the microtubule concerned. The minus end-directed tubule is indicated by arrows (to the left), and the plus end-directed tubule is indicate by arrowheads (to the right). Bar, 5 μm.
Figure 6
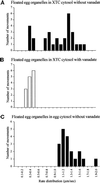
Analysis of the distribution of ER tubule rates in XTC and egg cytosols. (A) Floated egg organelles incubated in XTC cytosol displayed two apparent peaks of of ER extension rates: a slow rate at 0.3–0.5 μm/s, and a faster rate of between 0.7 and 1.8 μm/s. (B) When 20 μM vanadate was included, the faster peak of rates was abolished, but a slower peak of between 0.2 and 0.5 μm/s remained. (C) In egg cytosol, only a single peak of rates of egg ER motility at between 1.0 and 2.0 μm/s was observed (all motility was abolished by vanadate).
Figure 7
Plus end-directed ER motility in XTC cytosol is inhibited by the function-blocking anti-KHC antibody SUK4. Floated egg ER membranes were preincubated either with mouse IgG (control antibody) or with SUK4 and then mixed with XTC cytosol in the presence and absence of 20 μM vanadate. Motility was recorded in 10 random microscope fields of 2 min (mean of three independent assays). In the absence of vanadate, membrane motility was reduced by SUK4 pretreatment (compare IgG-treated control with SUK4-treated control). When vanadate was included, motility of SUK4-treated ER was abolished, suggesting that the vanadate-insensitive component of egg ER motility in XTC cytosol is driven by conventional kinesin. Bars indicate SEs.
Figure 8
Analysis of ER-associated motors after incubation in egg and XTC cytosols. Floated egg ER membranes were incubated in A/S buffer, egg cytosol, or XTC cytosol and then recovered again by flotation and analyzed by immunoblotting. Lane 1, egg cytosolic proteins; lane 2, floated egg ER membranes; lane 3, egg ER after incubation in A/S buffer; lane 4, egg ER after incubation in egg cytosol; lane 5, egg ER after incubation in XTC cytosol; lane 6, XTC cytosolic proteins. In each lane, equal quantities of proteins were loaded, and membranes were probed with an antibody against a subunit of dynactin complex (p150Glued), antibodies against dynein intermediate chain (DIC; clone IC74), and KHC. This experiment was carried out three times, and on each occasion the same results were obtained.
Similar articles
- Role of kinesin-1 and cytoplasmic dynein in endoplasmic reticulum movement in VERO cells.
Woźniak MJ, Bola B, Brownhill K, Yang YC, Levakova V, Allan VJ. Woźniak MJ, et al. J Cell Sci. 2009 Jun 15;122(Pt 12):1979-89. doi: 10.1242/jcs.041962. Epub 2009 May 19. J Cell Sci. 2009. PMID: 19454478 Free PMC article. - Cell cycle regulation of dynein association with membranes modulates microtubule-based organelle transport.
Niclas J, Allan VJ, Vale RD. Niclas J, et al. J Cell Biol. 1996 May;133(3):585-93. doi: 10.1083/jcb.133.3.585. J Cell Biol. 1996. PMID: 8636233 Free PMC article. - Kinesin is the motor for microtubule-mediated Golgi-to-ER membrane traffic.
Lippincott-Schwartz J, Cole NB, Marotta A, Conrad PA, Bloom GS. Lippincott-Schwartz J, et al. J Cell Biol. 1995 Feb;128(3):293-306. doi: 10.1083/jcb.128.3.293. J Cell Biol. 1995. PMID: 7844144 Free PMC article. - Dynamic phase microscopy reveals periodic oscillations of endoplasmic reticulum during network formation.
Vyshenskaya TV, Tychinsky VP, Weiss DG, Kuznetsov SA. Vyshenskaya TV, et al. Biochemistry (Mosc). 2014 Sep;79(9):907-16. doi: 10.1134/S0006297914090077. Biochemistry (Mosc). 2014. PMID: 25385018 Review. - Isolation and analysis of microtubule motor proteins.
Saxton WM. Saxton WM. Methods Cell Biol. 1994;44:279-88. doi: 10.1016/s0091-679x(08)60919-x. Methods Cell Biol. 1994. PMID: 7707957 Review.
Cited by
- Tubulin Isotypes: Emerging Roles in Defining Cancer Stem Cell Niche.
Maliekal TT, Dharmapal D, Sengupta S. Maliekal TT, et al. Front Immunol. 2022 May 26;13:876278. doi: 10.3389/fimmu.2022.876278. eCollection 2022. Front Immunol. 2022. PMID: 35693789 Free PMC article. Review. - Regulation of melanosome movement in the cell cycle by reversible association with myosin V.
Rogers SL, Karcher RL, Roland JT, Minin AA, Steffen W, Gelfand VI. Rogers SL, et al. J Cell Biol. 1999 Sep 20;146(6):1265-76. doi: 10.1083/jcb.146.6.1265. J Cell Biol. 1999. PMID: 10491390 Free PMC article. - Dynamic microtubules and endomembrane cycling contribute to polarity establishment and early development of Ectocarpus mitospores.
Green JJ, Cordero Cervantes D, Peters NT, Logan KO, Kropf DL. Green JJ, et al. Protoplasma. 2013 Oct;250(5):1035-43. doi: 10.1007/s00709-012-0476-5. Epub 2013 Jan 16. Protoplasma. 2013. PMID: 23322087 - Phosphorylation controls CLIMP-63-mediated anchoring of the endoplasmic reticulum to microtubules.
Vedrenne C, Klopfenstein DR, Hauri HP. Vedrenne C, et al. Mol Biol Cell. 2005 Apr;16(4):1928-37. doi: 10.1091/mbc.e04-07-0554. Epub 2005 Feb 9. Mol Biol Cell. 2005. PMID: 15703217 Free PMC article. - Dynein light intermediate chains maintain spindle bipolarity by functioning in centriole cohesion.
Jones LA, Villemant C, Starborg T, Salter A, Goddard G, Ruane P, Woodman PG, Papalopulu N, Woolner S, Allan VJ. Jones LA, et al. J Cell Biol. 2014 Nov 24;207(4):499-516. doi: 10.1083/jcb.201408025. J Cell Biol. 2014. PMID: 25422374 Free PMC article.
References
- Allan V. Assay of membrane motility in interphase and metaphase Xenopus extracts. In: Scholey JM, editor. Methods in Cell Biology. San Diego: Academic Press; 1993. pp. 203–226. - PubMed
- Allan V. Role of motor proteins in organizing the endoplasmic reticulum and Golgi apparatus. Semin Cell Dev Biol. 1996;7:335–342.
- Allan V, Vale R. Movement of membrane tubules along microtubules in vitro: evidence for specialized sites of motor attachment. J Cell Sci. 1994;107:1885–1897. - PubMed
- Allan VJ. Organelle motility and membrane network formation in metaphase and interphase cell-free extracts. Methods Enzymol. 1998;298:339–353. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources