The telomerase RNA pseudoknot is critical for the stable assembly of a catalytically active ribonucleoprotein - PubMed (original) (raw)
The telomerase RNA pseudoknot is critical for the stable assembly of a catalytically active ribonucleoprotein
D Gilley et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Telomerase is a ribonucleoprotein reverse transcriptase that synthesizes telomeric DNA. A pseudoknot structure is phylogenetically conserved within the RNA component of telomerase in all ciliated protozoans examined. Here, we report that disruptions of the pseudoknot base pairing within the telomerase RNA from Tetrahymena thermophila prevent the stable assembly in vivo of an active telomerase. Restoring the base-pairing potential of the pseudoknot by compensatory changes restores telomerase activity to essentially wild-type levels. Therefore, the pseudoknot topology rather than sequence is critical for an active telomerase. Furthermore, we show that disruption of the pseudoknot prevents the association of the RNA with the reverse transcriptase protein subunit of telomerase. Thus, we provide an example of a structural motif within the telomerase RNA that is required for telomerase function and identify the domain that is required for telomerase complex formation. Hence, we identify a biological role for a pseudoknot: promoting the stable assembly of a catalytically active ribonucleoprotein.
Figures
Figure 1
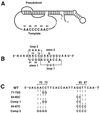
Ciliate TER structure and pseudoknot sequence alterations. (A) The conserved core structure of ciliate TER determined by phylogenetic covariation and structural probing (4), highlighting the pseudoknot domain that forms into a quasicontinuous helix on stacking of the two stems, and the wild-type template sequence from T. thermophila. (B) Detail of the pseudoknot domain from T. thermophila with sequence, position numbers, and standard designations of stems 1 and 2 as well as loops 1 and 2. (C) Sequence alterations used within the pseudoknot domain. The top line shows the wild-type pseudoknot RNA sequence from T. thermophila. Specific changes are shown directly below the wild-type residues that have been changed. Comp 1 and Comp 2, compensatory mutations 1 and 2.
Figure 2
Relative telomerase activity levels of disruptions and compensatory alterations within the pseudoknot domain compared with wild-type telomerase. Telomerase activity levels were normalized by using cell numbers and total protein content of partially purified preparations. The results represent an average of three separate experiments with a minimum of two lines for each sample. (A) Two-base pseudoknot disruptions, 71–72G and 84–85C, and the corresponding compensatory alteration, Comp 1, compared with activity levels for wild-type telomerase (WT). (B) Four-base pseudoknot disruption, 84–87C, and the corresponding compensatory alteration, Comp 2, compared with activity level for wild-type telomerase (WT).
Figure 3
Marking the template in combination with pseudoknot alterations. (A) Standard telomerase in vitro assay reactions separated on 10% PAGE. Cells were transformed with various pseudoknot wild-type or pseudoknot-altered TER genes that were marked at the template with the 43A template mutation. Lane 1, wild-type (WT) at pseudoknot; lane 2, Comp 1; lane 3, Comp 2; lane 4, 84–87C. (B) Schematic of 43A and wild-type templates. Telomerase with a 43A template change synthesizes a seven-base repeat consisting of G4T3. Wild-type telomerase synthesizes a six-base repeat consisting of G4T2. Note that cell numbers and protein content were not normalized between these different lines and that telomerase was harvested from cells in stationary phase culture.
Figure 4
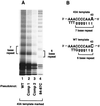
TER-associated protein complexes separated by native gel electrophoresis. (A) Northern analysis of native complexes. Northern blots probed with either a wild-type-specific end-labeled oligonucleotide (5′-ATTTGAACCTAATTGG-3′) that annealed only to wild-type TER (lanes 1–6) or a mutant-specific end-labeled oligonucleotide (5′-ATTTGGGGGTAATTGG-3′) that annealed only to 84–87C and Comp 2 TERs (lanes 7–12). These antisense oligonucleotides anneal to residues 92–77 of T. thermophila TER. Lane 1 and 7, wild-type; lane 2 and 8, 84–87C; lane 3 and 9, Comp 2; lane 4 and 10, wild-type with 43A template; lane 5 and 11, Comp 2 with 43A template; lane 6 and 12, 84–87C with 43A template. (B) Western analysis of native complexes. Western blots probed with antibody against p95 (lanes 13–18), p80 (lanes 19–24), or p133 (lanes 25–30). Lanes 13, 19, and 25, wild-type; lanes 14, 20, and 26, Comp 2; lanes 15, 21, and 27, 84–87C; lanes 16, 22, and 28, wild-type with 43A template; lanes 17, 23, and 29, Comp 2 with 43A template; lanes 18, 24, and 30, 84–87C.
Similar articles
- Structure and folding of the Tetrahymena telomerase RNA pseudoknot.
Cash DD, Feigon J. Cash DD, et al. Nucleic Acids Res. 2017 Jan 9;45(1):482-495. doi: 10.1093/nar/gkw1153. Epub 2016 Nov 29. Nucleic Acids Res. 2017. PMID: 27899638 Free PMC article. - Structural basis for telomerase RNA recognition and RNP assembly by the holoenzyme La family protein p65.
Singh M, Wang Z, Koo BK, Patel A, Cascio D, Collins K, Feigon J. Singh M, et al. Mol Cell. 2012 Jul 13;47(1):16-26. doi: 10.1016/j.molcel.2012.05.018. Epub 2012 Jun 14. Mol Cell. 2012. PMID: 22705372 Free PMC article. - The architecture of Tetrahymena telomerase holoenzyme.
Jiang J, Miracco EJ, Hong K, Eckert B, Chan H, Cash DD, Min B, Zhou ZH, Collins K, Feigon J. Jiang J, et al. Nature. 2013 Apr 11;496(7444):187-92. doi: 10.1038/nature12062. Epub 2013 Apr 3. Nature. 2013. PMID: 23552895 Free PMC article. - Structure and function of telomerase RNA.
Theimer CA, Feigon J. Theimer CA, et al. Curr Opin Struct Biol. 2006 Jun;16(3):307-18. doi: 10.1016/j.sbi.2006.05.005. Epub 2006 May 18. Curr Opin Struct Biol. 2006. PMID: 16713250 Review. - The unmasking of telomerase.
Legassie JD, Jarstfer MB. Legassie JD, et al. Structure. 2006 Nov;14(11):1603-9. doi: 10.1016/j.str.2006.09.004. Structure. 2006. PMID: 17098185 Review.
Cited by
- A human-Tetrahymena pseudoknot chimeric telomerase RNA reconstitutes a nonprocessive enzyme in vitro that is defective in telomere elongation.
Marie-Egyptienne DT, Cerone MA, Londoño-Vallejo JA, Autexier C. Marie-Egyptienne DT, et al. Nucleic Acids Res. 2005 Sep 28;33(17):5446-57. doi: 10.1093/nar/gki848. Print 2005. Nucleic Acids Res. 2005. PMID: 16192571 Free PMC article. - A universal telomerase RNA core structure includes structured motifs required for binding the telomerase reverse transcriptase protein.
Lin J, Ly H, Hussain A, Abraham M, Pearl S, Tzfati Y, Parslow TG, Blackburn EH. Lin J, et al. Proc Natl Acad Sci U S A. 2004 Oct 12;101(41):14713-8. doi: 10.1073/pnas.0405879101. Epub 2004 Sep 15. Proc Natl Acad Sci U S A. 2004. PMID: 15371596 Free PMC article. - Effect of antisense oligodeoxynucleotide of telomerase RNA on telomerase activity and cell apoptosis in human colon cancer.
Jiang YA, Luo HS, Fan LF, Jiang CQ, Chen WJ. Jiang YA, et al. World J Gastroenterol. 2004 Feb 1;10(3):443-5. doi: 10.3748/wjg.v10.i3.443. World J Gastroenterol. 2004. PMID: 14760776 Free PMC article. - YNMG tetraloop formation by a dyskeratosis congenita mutation in human telomerase RNA.
Theimer CA, Finger LD, Feigon J. Theimer CA, et al. RNA. 2003 Dec;9(12):1446-55. doi: 10.1261/rna.5152303. RNA. 2003. PMID: 14624001 Free PMC article. - The RNA Base-Pairing Problem and Base-Pairing Solutions.
Lu Z, Chang HY. Lu Z, et al. Cold Spring Harb Perspect Biol. 2018 Dec 3;10(12):a034926. doi: 10.1101/cshperspect.a034926. Cold Spring Harb Perspect Biol. 2018. PMID: 30510063 Free PMC article. Review.
References
- Blackburn E H. Annu Rev Biochem. 1992;61:113–129. - PubMed
- Zakian A M, Prescott D M. Trends Cell Biol. 1996;6:29–33. - PubMed
- Greider C W. Annu Rev Biochem. 1996;65:337–365. - PubMed
- Blackburn E H. In: Telomerase RNA Structure and Function. Simons R W, Grunberg-Manago M, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1998. pp. 669–693.
- Shay J W, Werbin H, Wright W E. Ciba Found Symp. 1997;211:148–155. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources