Rapid and reversible relocalization of heat shock factor 1 within seconds to nuclear stress granules - PubMed (original) (raw)
Rapid and reversible relocalization of heat shock factor 1 within seconds to nuclear stress granules
C Jolly et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Heat shock factor 1 (HSF1) is essential for the stress-induced expression of heat shock genes. On exposure to heat shock, HSF1 localizes within seconds to discrete nuclear granules. On recovery from heat shock, HSF1 rapidly dissipates from these stress granules to a diffuse nucleoplasmic distribution, typical of unstressed cells. Subsequent reexposure to heat shock results in the rapid relocalization of HSF1 to the same stress granules with identical kinetics. Although the appearance of HSF1 stress granules corresponds to the hyperphosphorylated, trimeric DNA-binding state of HSF1 and correlates temporally with the inducible transcription of heat shock genes, they are also present in heat-shocked mitotic cells that are devoid of transcription. This finding suggests a role for HSF1 stress granules as a nuclear compartment for the temporal regulation and spatial organization of HSF1 activity and reveals new features of the dynamics of nuclear organization.
Figures
Figure 1
Three-dimensional structure of HSF1 stress granules. Rotated views of the nucleus of a heat-shocked HeLa cell displaying characteristic HSF1 stress granules. Arrowheads correspond to large and small single globular units, and the arrow indicates a grape-like cluster of smaller globular structures. (Bar = 5 μm.)
Figure 2
The HSF1 protein is highly mobile within the granules. FRAP experiment on living HeLa cells transiently transfected with a HSF1–GFP construct and exposed at 42°C for 15 minutes. A portion of a stress granule was photobleached by a 1-minute exposure to the 488 nm laser line of the confocal microscope. Averaged images were acquired at different time points: before photobleaching (a), just after photobleaching (b), 20 seconds after photobleaching (c), 45 seconds after photobleaching (d), 1 minute 15 seconds after photobleaching (e), and 2 minutes 45 seconds after photobleaching (f). (Bar = 5 μm.)
Figure 3
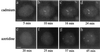
HSF1 granules are transient and reversible structures. Time-lapse microscopy during heat shock and recovery on living HeLa cells transiently transfected with a HSF1–GFP construct. Images were acquired before heat shock (a), after 30 seconds (b), 1 minute (c), and 3 minutes (d) of heat shock at 42°C, 2 minutes of recovery (e), 8 minutes of recovery (f), 40 minutes of recovery (g) and after 30 seconds (h), 1 minute (i), and 3 minutes (j) of a second heat shock at 42°C. (Bar = 5 μm.)
Figure 4
Kinetics of HSF1 granule formation during azetidine and cadmium treatments. Living HeLa cells transiently expressing a HSF1–GFP construct were exposed to 30 μM cadmium or to 5 mM azetidine, and images were acquired after 5 minutes (a), 10 minutes (b), 16 minutes (c), and 24 minutes (d) of cadmium treatment and after 20 minutes (e), 25 minutes (f), 37 minutes (g) and 45 minutes (h) of azetidine treatment. (Bars = 5 μm.)
Figure 5
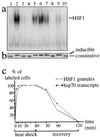
HSF1 granules and the activation of the heat shock response. Electrophoretic mobility-shift assay (a) and Western blot analysis (b) were performed on whole-cell extracts prepared from HeLa cells after various treatments: 37°C (lane 1), 1 hour at 42°C (lane 2), 1 hour at 42°C followed by 2 hours recovery at 37°C (lane 3), 1 hour at 42°C followed by 6 hours recovery at 37°C (lane 4), 1 hour at 42°C followed by 2 hours recovery at 4°C (lane 5), 1 hour at 42°C followed by 6 hours recovery at 4°C (lane 6), azetidine (5 mM) for 4 hours at 37°C (lane 7), azetidine (5 mM) for 4 hours at 4°C (lane 8), cadmium (30 μM) for 2 hours at 37°C (lane 9), and cadmium (30 μM) for 2 hours at 4°C (lane 10). (c) HSF1 granules were detected by immunofluorescence in HeLa cells, and hsp70 gene transcription was followed by detection of the nascent hsp70 transcripts by FISH. The percentages of cells displaying HSF1 granules (gray line) and hsp70 transcription sites (dashed line) were measured at different time points during exposure at 42°C and recovery.
Figure 6
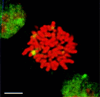
HSF1 granules are present in heat-shocked mitotic cells. HSF1 granules (green) were detected by immunofluorescence in mitotic HeLa cells after 5 minutes of heat shock at 42°C. DNA was counterstained with propidium iodide (red). (Bar = 5 μm.)
Similar articles
- HSF1 granules: a novel stress-induced nuclear compartment of human cells.
Cotto J, Fox S, Morimoto R. Cotto J, et al. J Cell Sci. 1997 Dec;110 ( Pt 23):2925-34. doi: 10.1242/jcs.110.23.2925. J Cell Sci. 1997. PMID: 9359875 - Changes in the number of HSF1 positive granules in the nucleus reflects heat shock semiquantitatively.
Nonaka T, Akimoto T, Mitsuhashi N, Tamaki Y, Nakano T. Nonaka T, et al. Cancer Lett. 2003 Dec 8;202(1):89-100. doi: 10.1016/s0304-3835(03)00481-6. Cancer Lett. 2003. PMID: 14643030 - Human heat shock factor 1 is predominantly a nuclear protein before and after heat stress.
Mercier PA, Winegarden NA, Westwood JT. Mercier PA, et al. J Cell Sci. 1999 Aug;112 ( Pt 16):2765-74. doi: 10.1242/jcs.112.16.2765. J Cell Sci. 1999. PMID: 10413683 - Nuclear stress bodies.
Biamonti G, Vourc'h C. Biamonti G, et al. Cold Spring Harb Perspect Biol. 2010 Jun;2(6):a000695. doi: 10.1101/cshperspect.a000695. Epub 2010 Apr 28. Cold Spring Harb Perspect Biol. 2010. PMID: 20516127 Free PMC article. Review. - Membrane-Bound Meet Membraneless in Health and Disease.
Zhang C, Rabouille C. Zhang C, et al. Cells. 2019 Aug 29;8(9):1000. doi: 10.3390/cells8091000. Cells. 2019. PMID: 31470564 Free PMC article. Review.
Cited by
- Inhibition of HSF1 and SAFB Granule Formation Enhances Apoptosis Induced by Heat Stress.
Watanabe K, Ohtsuki T. Watanabe K, et al. Int J Mol Sci. 2021 May 7;22(9):4982. doi: 10.3390/ijms22094982. Int J Mol Sci. 2021. PMID: 34067147 Free PMC article. - Human chromosomes 9, 12, and 15 contain the nucleation sites of stress-induced nuclear bodies.
Denegri M, Moralli D, Rocchi M, Biggiogera M, Raimondi E, Cobianchi F, De Carli L, Riva S, Biamonti G. Denegri M, et al. Mol Biol Cell. 2002 Jun;13(6):2069-79. doi: 10.1091/mbc.01-12-0569. Mol Biol Cell. 2002. PMID: 12058070 Free PMC article. - The ribosomal RNA processing machinery is recruited to the nucleolar domain before RNA polymerase I during Xenopus laevis development.
Verheggen C, Almouzni G, Hernandez-Verdun D. Verheggen C, et al. J Cell Biol. 2000 Apr 17;149(2):293-306. doi: 10.1083/jcb.149.2.293. J Cell Biol. 2000. PMID: 10769023 Free PMC article. - Optimal HSF1 activation in response to acute cold stress in BAT requires nuclear TXNIP.
Waldhart AN, Lau KH, Dykstra H, Avequin T, Wu N. Waldhart AN, et al. iScience. 2023 Apr 10;26(5):106538. doi: 10.1016/j.isci.2023.106538. eCollection 2023 May 19. iScience. 2023. PMID: 37168572 Free PMC article. - Integrated lncRNA function upon genomic and epigenomic regulation.
Herman AB, Tsitsipatis D, Gorospe M. Herman AB, et al. Mol Cell. 2022 Jun 16;82(12):2252-2266. doi: 10.1016/j.molcel.2022.05.027. Mol Cell. 2022. PMID: 35714586 Free PMC article. Review.
References
- Lindquist S. Annu Rev Biochem. 1986;55:1151–1191. - PubMed
- Morimoto R I, Tissières A, Georgopoulos C. The Biology of Heat Shock Proteins and Molecular Chaperones. Plainview, NY: Cold Spring Harbor Lab. Press; 1994.
- Wu C. Annu Rev Cell Dev Biol. 1995;11:441–469. - PubMed
- Morimoto R I. Genes Dev. 1998;12:3788–3796. - PubMed
- Westwood J T, Clos J, Wu C. Nature (London) 1991;353:822–827. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases