"Global" cell replacement is feasible via neural stem cell transplantation: evidence from the dysmyelinated shiverer mouse brain - PubMed (original) (raw)
"Global" cell replacement is feasible via neural stem cell transplantation: evidence from the dysmyelinated shiverer mouse brain
B D Yandava et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Many diseases of the central nervous system (CNS), particularly those of genetic, metabolic, or infectious/inflammatory etiology, are characterized by "global" neural degeneration or dysfunction. Therapy might require widespread neural cell replacement, a challenge not regarded conventionally as amenable to neural transplantation. Mouse mutants characterized by CNS-wide white matter disease provide ideal models for testing the hypothesis that neural stem cell transplantation might compensate for defective neural cell types in neuropathologies requiring cell replacement throughout the brain. The oligodendrocytes of the dysmyelinated shiverer (shi) mouse are "globally" dysfunctional because they lack myelin basic protein (MBP) essential for effective myelination. Therapy, therefore, requires widespread replacement with MBP-expressing oligodendrocytes. Clonal neural stem cells transplanted at birth-using a simple intracerebroventricular implantation technique-resulted in widespread engraftment throughout the shi brain with repletion of MBP. Accordingly, of the many donor cells that differentiated into oligodendroglia-there appeared to be a shift in the fate of these multipotent cells toward an oligodendroglial fate-a subgroup myelinated up to 52% (mean = approximately 40%) of host neuronal processes with better compacted myelin of a thickness and periodicity more closely approximating normal. A number of recipient animals evinced decrement in their symptomatic tremor. Therefore, "global" neural cell replacement seems feasible for some CNS pathologies if cells with stem-like features are used.
Figures
Figure 1
NSCs can express MBP. Two engraftable NSC clones, known to give rise to oligodendrocytes in vivo after transplantation, are reacted with an antibody to MBP in vitro by using immunoperoxidase methodology. (A) A subpopulation of NSC clone C17.2 (arrows) differentiates into MBP-expressing cells after exposure to conditioned medium from a primary culture of newborn mouse forebrain. (B) Cells from clone C27.3 that spontaneously differentiated toward MBP expression. The present experiments were performed by using clone C17.2 because of prior experience with these cells in CNS-wide gene therapy engraftment paradigms.
Figure 2
NSCs engraft extensively throughout the shi dysmyelinated brain, including within white tracts. _LacZ_-expressing NSCs were transplanted into newborn shi mutants and analyzed systematically at intervals between 2 and 8 weeks after engraftment. (A) Semiserial coronal sections through the shi brain at adulthood demonstrate widely disseminated integration of blue X-gal+ donor-derived cells throughout the neuraxis. (B_–_D) Progressively higher magnification of donor-derived cell integration in white tracts (arrows) at 2 weeks of age.
Figure 3
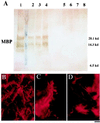
MBP expression in mature transplanted and control brains. (A) Western analysis for MBP in whole-brain lysates. The brains of three representative transplanted shi mutants (lanes 2–4) express MBP at levels close to that of an age-matched, unaffected mouse (lane 1, positive control) and significantly greater than the amounts seen in untransplanted (lanes 7 and 8, negative control) or unengrafted (lanes 5 and 6, negative control), age-matched shi mutants. (Identical total protein amounts were loaded in each lane.) (B_–_D) Immunocytochemical analysis for MBP. (B) The brain of a mature unaffected mouse is immunoreactive to an antibody to MBP (revealed with a Texas Red-conjugated secondary antibody). (C and D) Age-matched engrafted brains from shi mice similarly show immunoreactivity. Untransplanted shi brains lack MBP. Therefore, MBP immunoreactivity also classically has been a marker for normal, donor-derived oligodendrocytes (C and D) in transplant paradigms (31).
Figure 4
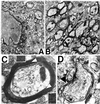
Engrafted NSCs in recipient shi mutants differentiate into oligodendrocytes. (A and B) Donor-derived X-gal+ cells in representative sections through the corpus callosum possess characteristic oligodendroglial features (small, round or polygonal cell bodies with multiple fine processes oriented in the direction of the neural fiber tracts). (C) Close-up of a representative donor-derived anti-β-gal immunoreactive oligodendrocyte (arrow) extending multiple processes toward and beginning to enwrap large, adjacent axonal bundles (“a”) viewed on end in a section through the corpus callosum. That cells such as those in A_–_C (and in Fig. 3 C and D) are oligodendroglia is confirmed by the representative electron micrograph in D (and in Fig. 5_A_), demonstrating the defining ultrastructural features detailed in Materials and Methods. A donor-derived X-gal-labeled oligodendrocyte (“LO”) can be distinguished by the electron-dense X-gal precipitate that typically is localized to the nuclear membrane (arrow), endoplasmic reticulum (arrowhead), and other cytoplasmic organelles. The area indicated by the arrowhead is magnified in the Inset to demonstrate the unique crystalline nature of individual precipitate particles.
Figure 5
NSC-derived “replacement” oligodendrocytes appear functional as demonstrated by ultrastructural evidence of myelination of shi axons. In regions of MBP-expressing NSC engraftment, shi neuronal processes become enwrapped by thick, better-compacted myelin. (A) At 2 weeks posttransplant, a representative donor-derived, labeled oligodendrocyte (“LO”) [recognized by extensive X-gal precipitate (“p”) in the nuclear membrane, cytoplasmic organelles, and processes] is extending processes (a representative one is delineated by arrowheads) to host neurites and is beginning to ensheathe them with myelin (“m”). (B) If engrafted shi regions, such as that in A, are followed over time (e.g., to 4 weeks of age as pictured here), the myelin begins to appear healthier, thicker, and better compacted (examples indicated by arrows) than that in age-matched, untransplanted control mutants. (C) By 6 weeks posttransplant, these mature into even thicker wraps; ≈40% of host axons are ensheathed by myelin (a higher-power view of a representative axon is illustrated in C) that is dramatically thicker and better compacted than that of shi myelin [an example of which is shown in D (black arrowhead) from an unengrafted region of an otherwise successfully engrafted shi brain]. In C, open arrowheads indicate representative regions of myelin that are magnified in the adjacent respective Insets; major dense lines are evident.
Figure 6
Functional and behavioral assessment of transplanted shi mutants and controls. The shi mutation is characterized by the onset of tremor and a “shivering gait” by the second to third postnatal week. The degree of motor dysfunction in animals was gauged in two ways: (i) by blindly scoring periods of standardized videotaped cage behavior of experimental and control animals and (ii) by measuring the amplitude of tail displacement from the body’s rostral–caudal axis (an objective, quantifiable index of tremor) (see Materials and Methods). Video freeze-frames of representative unengrafted and successfully engrafted shi mice are seen in A and B, respectively. The whole-body tremor and ataxic movement observed in the unengrafted symptomatic animal (A) causes the frame to blur, a contrast to the well focused frame of the asymptomatic transplanted shi mouse (B). The neurologic scoring of such mice is detailed in Materials and Methods and Table 1; 60% of transplanted mutants evinced nearly normal-appearing behavior as in B and attained scores that were not significantly different from normal controls. C and D depict the manner in which whole-body tremor was mirrored by the amplitude of tail displacement (hatched, gray arrow in C), measured perpendicularly from a line drawn in the direction of the animal’s movement (solid, gray arrow, which represents the body’s long axis) (see Materials and Methods). Measurements were made by permitting a mouse, whose tail had been dipped in India ink, to move freely in a straight line on a sheet of graph paper as shown. Large degrees of tremor cause the tail to make widely divergent ink marks away from the midline, representing the body’s axis (C). Absence of tremor allows the tail to make long, straight, uninterrupted ink lines on the paper congruent with the body’s axis (D). The distance between points of maximal tail displacement from the axis was measured and averaged for transplanted and untransplanted shi mutants and for unaffected controls (hatched, gray arrow). C shows data from a poorly engrafted mutant that did not improve with respect to tremor whereas D reveals lack of tail displacement in a successfully engrafted, now asymptomatic shi mutant. Overall, 64% of transplanted shi mice examined displayed at least a 50% decrement in the degree of tremor or “shiver.” Several showed zero displacement (see Table 1).
Similar articles
- Myelination of congenitally dysmyelinated spinal cord axons by adult neural precursor cells results in formation of nodes of Ranvier and improved axonal conduction.
Eftekharpour E, Karimi-Abdolrezaee S, Wang J, El Beheiry H, Morshead C, Fehlings MG. Eftekharpour E, et al. J Neurosci. 2007 Mar 28;27(13):3416-28. doi: 10.1523/JNEUROSCI.0273-07.2007. J Neurosci. 2007. PMID: 17392458 Free PMC article. - Transplantation of neural precursor cells into the dysmyelinated CNS of mutant mice deficient in the myelin-associated glycoprotein and Fyn tyrosine kinase.
Ader M, Schachner M, Bartsch U. Ader M, et al. Eur J Neurosci. 2001 Aug;14(3):561-6. doi: 10.1046/j.0953-816x.2001.01673.x. Eur J Neurosci. 2001. PMID: 11553306 - Platelet-derived growth factor-responsive neural precursors give rise to myelinating oligodendrocytes after transplantation into the spinal cords of contused rats and dysmyelinated mice.
Plemel JR, Chojnacki A, Sparling JS, Liu J, Plunet W, Duncan GJ, Park SE, Weiss S, Tetzlaff W. Plemel JR, et al. Glia. 2011 Dec;59(12):1891-910. doi: 10.1002/glia.21232. Epub 2011 Aug 23. Glia. 2011. PMID: 22407783 - The dysmyelinating mouse mutations shiverer (shi) and myelin deficient (shimld).
Readhead C, Hood L. Readhead C, et al. Behav Genet. 1990 Mar;20(2):213-34. doi: 10.1007/BF01067791. Behav Genet. 1990. PMID: 1693848 Review. - Remyelination: cellular and gene therapy.
Billinghurst LL, Taylor RM, Snyder EY. Billinghurst LL, et al. Semin Pediatr Neurol. 1998 Sep;5(3):211-28. doi: 10.1016/s1071-9091(98)80036-3. Semin Pediatr Neurol. 1998. PMID: 9777679 Review.
Cited by
- MR-based imaging of neural stem cells.
Politi LS. Politi LS. Neuroradiology. 2007 Jun;49(6):523-34. doi: 10.1007/s00234-007-0219-z. Epub 2007 Mar 8. Neuroradiology. 2007. PMID: 17345076 Review. - Advances in tissue engineering.
Langer R, Vacanti J. Langer R, et al. J Pediatr Surg. 2016 Jan;51(1):8-12. doi: 10.1016/j.jpedsurg.2015.10.022. Epub 2015 Nov 10. J Pediatr Surg. 2016. PMID: 26711689 Free PMC article. Review. - Glial progenitor cell-based treatment of the childhood leukodystrophies.
Osorio MJ, Goldman SA. Osorio MJ, et al. Exp Neurol. 2016 Sep;283(Pt B):476-88. doi: 10.1016/j.expneurol.2016.05.010. Epub 2016 May 8. Exp Neurol. 2016. PMID: 27170209 Free PMC article. Review. - Neural stem cell implantation extends life in Niemann-Pick C1 mice.
Ahmad I, Hunter RE, Flax JD, Snyder EY, Erickson RP. Ahmad I, et al. J Appl Genet. 2007;48(3):269-72. doi: 10.1007/BF03195222. J Appl Genet. 2007. PMID: 17666780 - Neural stem cell therapy for subacute and chronic ischemic stroke.
Boese AC, Le QE, Pham D, Hamblin MH, Lee JP. Boese AC, et al. Stem Cell Res Ther. 2018 Jun 13;9(1):154. doi: 10.1186/s13287-018-0913-2. Stem Cell Res Ther. 2018. PMID: 29895321 Free PMC article. Review.
References
- Snyder E Y, Taylor R M, Wolfe J H. Nature (London) 1995;374:367–370. - PubMed
- Lacorazza H D, Flax J D, Snyder E Y, Jendoubi M. Nat Med. 1996;4:424–429. - PubMed
- McKay R. Science. 1997;276:66–71. - PubMed
- Gage F H, Ray J, Fisher L J. Annu Rev Neurosci. 1995;18:159–192. - PubMed
- Gage F H, Christen Y, editors. Isolation, Characterization, and Utilization of CNS Stem Cells—Research and Perspectives in Neuroscience. Berlin: Springer; 1997.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous