The human papillomavirus type 16 E6 gene alone is sufficient to induce carcinomas in transgenic animals - PubMed (original) (raw)
The human papillomavirus type 16 E6 gene alone is sufficient to induce carcinomas in transgenic animals
S Song et al. J Virol. 1999 Jul.
Abstract
High-risk human papillomaviruses (HPVs) are the causative agents of certain human cancers. HPV type 16 (HPV16) is the papillomavirus most frequently associated with cervical cancer in women. The E6 and E7 genes of HPV are expressed in cells derived from these cancers and can transform cells in tissue culture. Animal experiments have demonstrated that E6 and E7 together cause tumors. We showed previously that E6 and E7 together or E7 alone could induce skin tumors in mice when these genes were expressed in the basal epithelia of the skin. In this study, we investigated the role that the E6 gene plays in carcinogenesis. We generated K14E6 transgenic mice, in which the HPV16 E6 gene was directed in its expression by the human keratin 14 promoter (hK14) to the basal layer of the epidermis. We found that E6 induced cellular hyperproliferation and epidermal hyperplasia and caused skin tumors in adult mice. Interestingly, the tumors derived from E6 were mostly malignant, as opposed to the tumors from E7 mice, which were mostly benign. This result leads us to hypothesize that E6 may contribute differently than E7 to HPV-associated carcinogenesis; whereas E7 primarily contributes to the early stages of carcinogenesis that lead to the formation of benign tumors, E6 primarily contributes to the late stages of carcinogenesis that lead to malignancy.
Figures
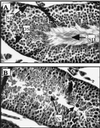
FIG. 1
Examples of skin sections analyzed for E6 mRNA by in situ hybridization with a specific antisense RNA probe. (A to C) Dark-field images (magnification, ×400) of skin sections from nontransgenic (A), K14E6 transgenic line 5718 (B) and K14E6 transgenic line 5737 (C) mice. Bright areas indicate strong hybridization to an E6-specific probe. Note that signal is brightest in the epidermis (ep) but also present in underlying epithelial structures (hair follicles) in the dermis (dm). (D) Bright-field image (×100) of an ear section from K14E6 line 5737. Dark areas in the epidermis of both sides of the ear indicate strong hybridization to the E6-specific probe. Note absence of signal in the underlying dermis and cartilage (ct).
FIG. 2
Histological and immunohistochemical analysis of epidermis from the ears of 6-week-old nontransgenic, K14E6 transgenic line 5737, p53-null, and E6/p53-null mice. All tissue sections were paraffin embedded. (A to D) Hematoxylin-and-eosin-stained sections of the ear at high magnification (×200). The epidermis is indicated by the arrow. Note thickening of the epidermis in the ears of K14E6 and K14E6/p53-null mice. Ear sections were stained immunohistochemically for PCNA (E to H) and BrdU (I to L). Positive cells are indicated by arrows. Note increased numbers of PCNA-positive and BrdU-positive cells and their appearance in the suprabasal portion of the epidermis in both K14E6 mice and K14E6/p53-null mice. In panels M to P, ear sections were stained for K14. Note thickening of the K14-positive portion of the epidermis in the K14E6 and K14E6/p53-null ear sections.
FIG. 3
Histological analysis of mouse testes. Normal mature sperm cells (sp), diploid spermatogonia stem cells (sg), and 4N spermatocytes in the seminiferous tubules from nontransgenic mice are indicated by arrows (A). Many giant cells (gc), not mature sperm cells, are seen in seminiferous tubules from K14E6 transgenic line 5743 mice (B).
FIG. 4
Histology of skin tumors derived from K14E6 transgenic mice. The K14E6 mice developed both benign and malignant skin tumors. (A) Section of papilloma, a benign outgrowth of skin with epidermal hyperplasia. The morphology of cells are relatively normal and the basement membrane in this lesion is intact (arrow). The epidermis and underlying dermis (arrowhead) are clearly demarcated. (B) Epidermoid carcinoma grade I. The cells form a concentric pattern that is poorly demarcated (arrowheads). In the center of these structures are keratinized and differentiated cells. Note the presence of “pearls” of keratin (arrow), characteristic of well-differentiated epidermoid carcinoma. (C) Example of grade III epidermoid carcinoma. The cells are more anaplastic than in panel B and do not form keratin pearls. These cells are still able to be keratinized individually (arrow).
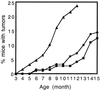
FIG. 5
Skin tumor development over 15 months of life in K14E6 line 5737 (●), line 5743 (■), and line 5737 homozygous (▴) mice. The numbers of mice monitored up to at least 12 months in the three groups were 181, 73, and 80, respectively.
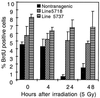
FIG. 6
Abrogation of radiation-induced growth arrest in the epidermis from K14E6 mice with low or high transgene copy number. Shown are percentages of BrdU-positive cells in the epidermis of K14E6 line 5737 mice carrying a high copy number of the transgene, K14E6 line 5718 mice carrying a low copy number of the transgene, and nontransgenic FVB/N mice that were treated or not treated with 5 Gy of ionizing radiation. Provided are the average values for three mice in each group ± 1 standard deviation. The 10-fold reduction in percentage of BrdU-positive cells in nontransgenic FVB/N mice is an indicator of the radiation-induced growth arrest. Note absence of reduction in the percentage of BrdU-positive cells in the epidermis from mice of both lines 5737 and 5718.
Similar articles
- Squamous epithelial hyperplasia and carcinoma in mice transgenic for the human papillomavirus type 16 E7 oncogene.
Herber R, Liem A, Pitot H, Lambert PF. Herber R, et al. J Virol. 1996 Mar;70(3):1873-81. doi: 10.1128/JVI.70.3.1873-1881.1996. J Virol. 1996. PMID: 8627712 Free PMC article. - Human papillomavirus types 16 E6 and E7 contribute differently to carcinogenesis.
Song S, Liem A, Miller JA, Lambert PF. Song S, et al. Virology. 2000 Feb 15;267(2):141-50. doi: 10.1006/viro.1999.0106. Virology. 2000. PMID: 10662610 - The PDZ ligand domain of the human papillomavirus type 16 E6 protein is required for E6's induction of epithelial hyperplasia in vivo.
Nguyen ML, Nguyen MM, Lee D, Griep AE, Lambert PF. Nguyen ML, et al. J Virol. 2003 Jun;77(12):6957-64. doi: 10.1128/jvi.77.12.6957-6964.2003. J Virol. 2003. PMID: 12768014 Free PMC article. - Basic mechanisms of high-risk human papillomavirus-induced carcinogenesis: roles of E6 and E7 proteins.
Narisawa-Saito M, Kiyono T. Narisawa-Saito M, et al. Cancer Sci. 2007 Oct;98(10):1505-11. doi: 10.1111/j.1349-7006.2007.00546.x. Epub 2007 Jul 23. Cancer Sci. 2007. PMID: 17645777 Free PMC article. Review. - Cellular targets of the oncoproteins encoded by the cancer associated human papillomaviruses.
Howley PM, Münger K, Romanczuk H, Scheffner M, Huibregtse JM. Howley PM, et al. Princess Takamatsu Symp. 1991;22:239-48. Princess Takamatsu Symp. 1991. PMID: 1668886 Review.
Cited by
- PATJ, a tight junction-associated PDZ protein, is a novel degradation target of high-risk human papillomavirus E6 and the alternatively spliced isoform 18 E6.
Storrs CH, Silverstein SJ. Storrs CH, et al. J Virol. 2007 Apr;81(8):4080-90. doi: 10.1128/JVI.02545-06. Epub 2007 Feb 7. J Virol. 2007. PMID: 17287269 Free PMC article. - Estrogen contributes to the onset, persistence, and malignant progression of cervical cancer in a human papillomavirus-transgenic mouse model.
Brake T, Lambert PF. Brake T, et al. Proc Natl Acad Sci U S A. 2005 Feb 15;102(7):2490-5. doi: 10.1073/pnas.0409883102. Epub 2005 Feb 7. Proc Natl Acad Sci U S A. 2005. PMID: 15699322 Free PMC article. - Efficacy of Topically Administered Dihydroartemisinin in Treating Papillomavirus-Induced Anogenital Dysplasia in Preclinical Mouse Models.
Gunder LC, Blaine-Sauer S, Johnson HR, Shin MK, Auyeung AS, Zhang W, Leverson GE, Ward-Shaw ET, King RE, McGregor SM, Matkowskyj KA, Lambert PF, Carchman EH. Gunder LC, et al. Viruses. 2022 Jul 26;14(8):1632. doi: 10.3390/v14081632. Viruses. 2022. PMID: 35893697 Free PMC article. - Gene expression profiles are altered in human papillomavirus-16 E6 D25E-expressing cell lines.
Jang M, Rhee JE, Jang DH, Kim SS. Jang M, et al. Virol J. 2011 Sep 25;8:453. doi: 10.1186/1743-422X-8-453. Virol J. 2011. PMID: 21943319 Free PMC article. - HPV16-E6 Oncoprotein Activates TGF-β and Wnt/_β_-Catenin Pathways in the Epithelium-Mesenchymal Transition of Cataracts in a Transgenic Mouse Model.
Rodríguez-Uribe G, Serafín-Higuera N, Damian-Morales G, Cortés-Malagón EM, García-Hernández V, Verdejo-Torres O, Campos-Blázquez JP, Trejo-Muñoz CR, Contreras RG, Ocadiz-Delgado R, Palacios-Reyes C, Lambert PF, Griep AE, Mancilla-Percino T, Escobar-Herrera J, Álvarez-Ríos E, Ugarte-Briones C, Moreno J, Gariglio P, Bonilla-Delgado J. Rodríguez-Uribe G, et al. Biomed Res Int. 2018 May 16;2018:2847873. doi: 10.1155/2018/2847873. eCollection 2018. Biomed Res Int. 2018. PMID: 29888254 Free PMC article.
References
- Anwar K, Nakakuki K, Naiki H, Inuzuka M. ras gene mutations and HPV infection are common in human laryngeal carcinoma. Int J Cancer. 1993;53:22–28. - PubMed
- Boyer S N, Wazer D E, Band V. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res. 1996;56:4620–4624. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials