Human herpesvirus 6 open reading frame U83 encodes a functional chemokine - PubMed (original) (raw)
Human herpesvirus 6 open reading frame U83 encodes a functional chemokine
P Zou et al. J Virol. 1999 Jul.
Abstract
Some viruses including herpesviruses have undergone evolution to benefit viral infection and propagation by pirating and modifying host genes such as chemokine genes. Human herpesvirus 6 (HHV-6), acutely or persistently infects mononuclear cells in vitro. DNA sequence analysis of HHV-6 has revealed that the putative protein encoded by an open reading frame (ORF) of the U83 gene in HHV-6 variant B resembled a human chemokine. We have cloned the U83 gene and analyzed the biological function of this gene. The U83 gene contained an ORF encoding a 113-amino-acid peptide, starting at the first methionine and containing a possible signal peptide and the typical cysteine residues characteristic of the chemokines. Reverse transcription-PCR analysis of mRNA and immunofluorescent-antibody testing of infected cells both indicated that the encoded protein was a late protein. The ORF U83 gene fused to the Fc gene was expressed as a fusion protein in COS-7 cells by transfection, and the fusion protein was purified from the supernatant of transfected cells to test its biological function. The purified protein was capable of inducing transient calcium mobilization in THP-1 cells and of chemotactically activating THP-1 cells. These findings suggested that the U83 protein might play an important role in HHV-6 propagation in vivo by activating and trafficking mononuclear cells to sites of viral replication, thus aiding the development of superbly efficient virus production mechanisms.
Figures
FIG. 1
Alignment of the HHV-6BU83 cDNA sequence and its deduced amino acid sequence. The 5′ and 3′ ends of the U83 cDNA were obtained by RACE. The U83 cDNA contained an ORF encoding 113 amino acids, starting at the first methionine codon, which contains the five cysteine residues (asterisks). The vertical arrow indicates the experimentally determined cleavage site of the signal sequence. The putative N-glycosylation site NAS is indicated by a dotted underline. The AATAAA sequence in the 3′ noncoding region indicates the putative polyadenylation signal, and the ATTTA is the mRNA destabilization signal that is often found among cytokine and chemokine cDNAs.
FIG. 2
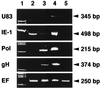
RT-PCR assay of the transcripts in the HST-infected cells treated with CHX and PFA. Total RNAs purified from mock-infected (lane 5) and HST-infected (lanes 2 to 4) CBMCs, which had been treated with CHX for 24 h (lane 2) or PFA for 24 h (lane 3) or left untreated (lane 4), were used for the RT-PCR assay of mRNA expression of HHV-6 IE-1, Pol, gH, U83, and cellular EF genes. The EF band, which was amplified from the endogeneous cellular control RNA, was expressed at approximately equal intensities in all lanes. In the presence of CHX or PFA, the U83 band was not detected. In the untreated sample in lane 4, the U83 band was detected. The results indicate that U83 is expressed as a late gene. Lane 1 shows molecular size markers.
FIG. 3
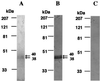
The monospecific antiserum specifically recognized HHV-6B U83 protein by Western blotting. Partially purified U83 protein from supernatants of rU83 virus-infected Hi-5 cell cultures was reacted with antiserum against GST-U83 fusion protein (A) or with preimmune serum (B).
FIG. 4
Immunofluorescence micrographs. (A) Mock-infected MT4 cells. (B) HST-infected MT4 cells stained with preimmune rabbit sera. (C) HST-infected MT4 cells stained with rabbit anti-U83 sera. (D to F) HST-infected MT4 cells cultured for 48 h in the presence of PFA (200 μg/ml) and stained with rabbit anti-U89 sera (D), monoclonal antibody OHV-2 for U41 (E), and rabbit anti-U83 sera (F).
FIG. 5
(A) U83-Fc proteins were purified from culture supernatants of transfected COS-7 cells by protein A affinity chromatography, subjected to electrophoresis on a 10% polyacrylamide gel, and stained with Coomassie brilliant blue. (B and C) Western blot analysis showed that purified U83-Fc proteins were detected with anti-U83 serum (B) but not with preimmune serum (C). Amino acid sequencing demonstrated that the NH2 termini of both the 38- and 40-kDa U83-Fc proteins started at Phe-21 of the predicted sequence. These results agreed with the putative signal cleavage site and molecular mass of the cleaved mature protein. Positions of size markers (in kilodaltons) are shown on the left.
FIG. 6
THP-1 cells were loaded with Indo-1 AM and stimulated with U83-Fc (100 nM), Fas-Fc (100 nM), or RANTES (100 nM). The arrows indicate the time of application. Intercellular concentrations of calcium were monitored by measuring the fluorescence ratio. U83 protein induced a calcium flux in THP-1 cells.
FIG. 7
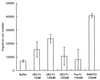
Chemotaxis of THP-1 cells by U83-Fc protein. THP-1 cell migration was measured in response to increased concentrations of U83-Fc protein (10, 100, and 200 nM) by using the transwell migration assay system (see Materials and Methods). RANTES at 100 nM served as a positive control. Each experiment was performed at least three times. Representative data from the three experiments are shown. The data are expressed as means and standard deviations.
Similar articles
- Relationship between U83 gene variation in human herpesvirus 6 and secretion of the U83 gene product.
Sjahril R, Isegawa Y, Tanaka T, Nakano K, Yoshikawa T, Asano Y, Ohshima A, Yamanishi K, Sugimoto N. Sjahril R, et al. Arch Virol. 2009;154(2):273-83. doi: 10.1007/s00705-008-0307-3. Epub 2009 Jan 20. Arch Virol. 2009. PMID: 19153640 - Novel, nonconsensus cellular splicing regulates expression of a gene encoding a chemokine-like protein that shows high variation and is specific for human herpesvirus 6.
French C, Menegazzi P, Nicholson L, Macaulay H, DiLuca D, Gompels UA. French C, et al. Virology. 1999 Sep 15;262(1):139-51. doi: 10.1006/viro.1999.9875. Virology. 1999. PMID: 10489348 - The equine herpesvirus 2 E1 open reading frame encodes a functional chemokine receptor.
Camarda G, Spinetti G, Bernardini G, Mair C, Davis-Poynter N, Capogrossi MC, Napolitano M. Camarda G, et al. J Virol. 1999 Dec;73(12):9843-8. doi: 10.1128/JVI.73.12.9843-9848.1999. J Virol. 1999. PMID: 10559296 Free PMC article. - [Genes and the gene products encoded by HHV-6 and HHV-7].
Mori Y. Mori Y. Nihon Rinsho. 2006 Mar;64 Suppl 3:386-93. Nihon Rinsho. 2006. PMID: 16615503 Review. Japanese. No abstract available. - Molecular biology of human herpesviruses 6A and 6B.
Inoue N, Dambaugh TR, Pellett PE. Inoue N, et al. Infect Agents Dis. 1993 Dec;2(6):343-60. Infect Agents Dis. 1993. PMID: 8012736 Review.
Cited by
- Molecular machinations: chemokine signals in host-pathogen interactions.
Chensue SW. Chensue SW. Clin Microbiol Rev. 2001 Oct;14(4):821-35, table of contents. doi: 10.1128/CMR.14.4.821-835.2001. Clin Microbiol Rev. 2001. PMID: 11585787 Free PMC article. Review. - International Union of Basic and Clinical Pharmacology. [corrected]. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors.
Bachelerie F, Ben-Baruch A, Burkhardt AM, Combadiere C, Farber JM, Graham GJ, Horuk R, Sparre-Ulrich AH, Locati M, Luster AD, Mantovani A, Matsushima K, Murphy PM, Nibbs R, Nomiyama H, Power CA, Proudfoot AE, Rosenkilde MM, Rot A, Sozzani S, Thelen M, Yoshie O, Zlotnik A. Bachelerie F, et al. Pharmacol Rev. 2013 Nov 11;66(1):1-79. doi: 10.1124/pr.113.007724. Print 2014. Pharmacol Rev. 2013. PMID: 24218476 Free PMC article. Review. - Murine cytomegalovirus CC chemokine homolog MCK-2 (m131-129) is a determinant of dissemination that increases inflammation at initial sites of infection.
Saederup N, Aguirre SA, Sparer TE, Bouley DM, Mocarski ES. Saederup N, et al. J Virol. 2001 Oct;75(20):9966-76. doi: 10.1128/JVI.75.20.9966-9976.2001. J Virol. 2001. PMID: 11559829 Free PMC article. - Implication of human herpesviruses in oncogenesis through immune evasion and supression.
Alibek K, Baiken Y, Kakpenova A, Mussabekova A, Zhussupbekova S, Akan M, Sultankulov B. Alibek K, et al. Infect Agent Cancer. 2014 Jan 20;9(1):3. doi: 10.1186/1750-9378-9-3. Infect Agent Cancer. 2014. PMID: 24438207 Free PMC article. - Differential tropism of human herpesvirus 6 (HHV-6) variants and induction of latency by HHV-6A in oligodendrocytes.
Ahlqvist J, Fotheringham J, Akhyani N, Yao K, Fogdell-Hahn A, Jacobson S. Ahlqvist J, et al. J Neurovirol. 2005 Aug;11(4):384-94. doi: 10.1080/13550280591002379. J Neurovirol. 2005. PMID: 16162481 Free PMC article.
References
- Ablashi D V, Balachandran N, Josephs S F, Hung C L, Krueger G R, Kramarsky B, Salahuddin S Z, Gallo R C. Genomic polymorphism, growth properties, and immunologic variations in human herpesvirus-6 isolates. Virology. 1991;184:545–552. - PubMed
- Aubin J T, Agut H, Collandre H, Yamanishi K, Chandran B, Montagnier L, Huraux J M. Antigenic and genetic differentiation of the two putative types of human herpes virus 6. J Virol Methods. 1993;41:223–234. - PubMed
- Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. - PubMed
- Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases