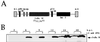Induction of apoptosis by double-stranded-RNA-dependent protein kinase (PKR) involves the alpha subunit of eukaryotic translation initiation factor 2 and NF-kappaB - PubMed (original) (raw)
Induction of apoptosis by double-stranded-RNA-dependent protein kinase (PKR) involves the alpha subunit of eukaryotic translation initiation factor 2 and NF-kappaB
J Gil et al. Mol Cell Biol. 1999 Jul.
Abstract
The double-stranded (ds) RNA-dependent protein kinase (PKR) is a key mediator of antiviral effects of interferon (IFN) and an active player in apoptosis induced by different stimuli. The translation initiation factor eIF-2alpha (alpha subunit of eukaryotic translation initiation factor 2) and IkappaBalpha, the inhibitor of the transcription factor NF-kappaB, have been proposed as downstream mediators of PKR effects. To evaluate the involvement of NF-kappaB and eIF-2alpha in the induction of apoptosis by PKR, we have used vaccinia virus (VV) recombinants that inducibly express PKR concomitantly with a dominant negative mutant of eIF-2alpha or a repressor form of IkappaBalpha. We found that while expression of PKR by a VV vector resulted in extensive inhibition of protein synthesis and induction of apoptosis, coexpression of PKR with a dominant negative mutant of eIF-2alpha (Ser-51-->Ala) reversed both the PKR-mediated translational block and PKR-induced apoptosis. Coexpression of PKR with a repressor form of IkappaBalpha (Ser-32, 36-Ala) also leads to the inhibition of apoptosis by abolishing NF-kappaB induction, while translation remains blocked. Treating cells with two different proteasome inhibitors which block IkappaBalpha degradation, prevented PKR-induced apoptosis, supporting results from coexpression studies. Biochemical analysis and transient assays revealed that PKR expression by a VV vector induced NF-kappaB binding and transactivation. In addition, upregulation of Fas mRNA transcription occurred during PKR activation. Our findings provide direct evidence for the involvement of eIF-2alpha and NF-kappaB in the induction of apoptosis by PKR.
Figures
FIG. 1
Inducible expression of a dominant negative eIF-2α mutant form by a VV recombinant. (A) Map of the pPR35 derived peIF-2α NP (S51A). The plasmid contains an IPTG-inducible copy of eIF-2α NP (S51A) under the regulation of a hybrid promoter consisting of the VV p4b promoter fused to two lacI operator (op) units. The plasmid also contains the lacI repressor gene under the control of the VV p7.5 promoter and was used to obtain recombinant VV by homologous recombination in TK− 143B cells. (B) Time course analysis of IPTG-dependent expression of eIF-2α NP by the VV eIF-2α NP. BSC-40 cells were infected with 4 PFU of the virus per cell and were treated with or without 1.5 mM IPTG, scraped, and collected at the indicated times postinfection. Extracts were lysed, and equal amounts of protein as determined by BCA were analyzed by SDS-PAGE in conjunction with Western blotting with an eIF-2α monoclonal antibody.
FIG. 2
VV eIF-2α NP rescues translation inhibition and prevents PKR-induced apoptosis. (A) BSC-40 cells grown in 12-well plates were infected at a total of 9 PFU/cell with the viruses indicated in the presence of 5 mM IPTG unless stated otherwise, transfected 1 h later with 0.5 μg of pPR15, and harvested at 24 hpi for the determination of apoptosis. Mean values of triplicates of the determined absorbance at 405 nm with standard deviations are given. Columns: 1, 3 PFU of VV PKR and 6 PFU of VV per cell; 2, 3 PFU of VV PKR, 3 PFU of VV eIF-2α NP, and 3 PFU of VV per cell; 3, 3 PFU of VV PKR and 6 PFU of VV eIF-2α NP per cell; 4, 6 PFU of VV eIF-2α NP and 3 PFU of VV per cell, without adding IPTG; 5, 6 PFU of VV eIF-2α NP and 3 PFU of VV per cell, but with IPTG; 6, 9 PFU of VV per cell. (B) Samples from the same experiment were harvested and lysed in buffer for luciferase determination as described in Materials and Methods. Mean values of triplicates with the standard deviations are given of the relative luciferase units measured in equal extracts. (C) Immunoblot analysis of PKR and eIF-2α NP of the same samples used in panels A and B.
FIG. 3
PKR mediates pIC-induced phosphorylation of IκBα on serine 32. PKR0/0 and PKR+/+ cells grown in 6-well plates were serum starved for 3 h and then transfected with 7 μg of pIC per well with Lipofectamine or just transfected with Lipofectamine as a mock negative control (M). Cells were collected 30 min posttreatment. For the PSI–TNF-α treatment (referred to as TNF-α), cells were serum starved for 2 h, and then 60 μM PSI was added for one additional hour before treatment with 50 ng of TNF-α per ml during 3 h in the presence of 60 μM PSI in order to achieve the accumulation of the serine 32-phosphorylated form of I-κBα. At the indicated times, the cells were washed with PBS and lysed in the plates by adding 200 μl of 1× Laemmli buffer, collected, cooled on ice, and sonicated twice for 10 s. Clarified extracts were separated by SDS–12% PAGE, transferred to nitrocellulose membrane, and immunoblotted with antibodies against phosphoserine 32 IκBα (upper panel) and β-actin (lower panel).
FIG. 4
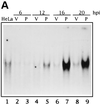
PKR expression from a VV vector induces NF-κB binding and transactivation. (A) Time course of NF-κB binding induced by PKR. HeLa cells were infected with 8 PFU of wild-type VV (lanes V) per cell or 4 PFU of VV PKR plus 4 PFU of VV per cell in the presence of 5 mM IPTG (lanes P) for the times indicated. As a control, HeLa cells were mock infected for 20 h. Nuclear extracts were analyzed by gel shift with a [α-32P]dCTP-labeled double-stranded probe containing two κB consensus sites. For the analysis of PKR-induced NF-κB-dependent transactivation, HeLa cells seeded in 6-well plates were mock infected or infected with 5 PFU of VV or VV PKR per cell. After 1 h of viral adsorption, 5 mM IPTG was added, and the cells were transfected with 2 μg of 3enh-ïB-ConA-luc vector (B) or LTR-luciferase plasmid (C) per well for an additional 5 h. Cells were collected at the indicated times postinfection and lysed for the luciferase determination. Duplicate experiments were performed.
FIG. 4
PKR expression from a VV vector induces NF-κB binding and transactivation. (A) Time course of NF-κB binding induced by PKR. HeLa cells were infected with 8 PFU of wild-type VV (lanes V) per cell or 4 PFU of VV PKR plus 4 PFU of VV per cell in the presence of 5 mM IPTG (lanes P) for the times indicated. As a control, HeLa cells were mock infected for 20 h. Nuclear extracts were analyzed by gel shift with a [α-32P]dCTP-labeled double-stranded probe containing two κB consensus sites. For the analysis of PKR-induced NF-κB-dependent transactivation, HeLa cells seeded in 6-well plates were mock infected or infected with 5 PFU of VV or VV PKR per cell. After 1 h of viral adsorption, 5 mM IPTG was added, and the cells were transfected with 2 μg of 3enh-ïB-ConA-luc vector (B) or LTR-luciferase plasmid (C) per well for an additional 5 h. Cells were collected at the indicated times postinfection and lysed for the luciferase determination. Duplicate experiments were performed.
FIG. 5
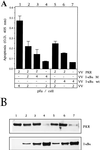
Lactacystin and PSI treatments prevent apoptosis of VV PKR-infected cells. (A) BSC-40 cells grown in 12-well plates were infected at a multiplicity of infection (MOI) of 6 with VV PKR in the presence of 5 mM IPTG with 3 PFU of VT7 and 3 PFU of VV RL per cell or 6 PFU of VV per cell and treated for 24 h with the indicated amounts of lactacystin. The cells were harvested at 24 hpi for the determination of apoptosis as previously described. Results are presented as the fold induction of apoptosis over that for VV-infected cells. (B) BSC-40 cells grown in 12-well plates were infected at a total MOI of 6 with VV PKR in the presence of 5 mM IPTG, with 3 PFU each of VT7 and VV RNase L per cell or with 6 PFU of VV per cell and treated for 24 h with the indicated amounts of PSI. The cells were harvested at 24 hpi for the determination of apoptosis. Results are presented as the fold induction of apoptosis over that for VV-infected cells. Extracts of the lactacystin treatment (C) and PSI treatment (D) experiments were separated by SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted with antibodies against RNase L and PKR. All of the ELISA experiments are represented as mean values of triplicate experiments.
FIG. 6
VV IκBα M expresses in an IPTG-dependent way a tagged mutant form of IκBα. (A) Map of the pPR35 derived pIκBα M (S32, 36A). The plasmid contains an IPTG-inducible copy of IκBα M (S32, 36A) fused to the SV5 tag in its C terminus under the regulation of a hybrid promoter consisting of the VV p4b promoter fused to two lacI operator (op) units. The plasmid also contains the lacI repressor gene under the control of the VV p7.5 promoter and was used to obtain recombinant VV by homologous recombination in TK− 143B cells. (B) Western blot time course analysis of IPTG-dependent expression of IκBα M by VV IκBα M. BSC-40 cells were infected at an MOI of 4 with the virus and treated with or without 1.5 mM IPTG; they were then scraped and collected at the indicated times postinfection. Extracts were lysed, and equal amounts of protein as determined by BCA were analyzed by SDS-PAGE in conjunction with Western blotting with anti-SV5 monoclonal antibody.
FIG. 7
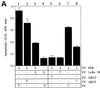
Coinfection of VV PKR with VV IκBα M inhibits PKR-induced apoptosis. (A) BSC-40 cells grown in 12-well plates were infected at an MOI of 10 with the viruses indicated in the presence of 5 mM IPTG and harvested at 24 hpi to determine the absorbance at 405 nm. The mean values of triplicate experiments with standard deviations are given. Columns: 1, 5 PFU of VV PKR and 5 PFU of VV per cell; 2, 5 PFU of VV PKR and 5 PFU of VV aBcl-2 per cell; 3, 5 PFU of VV PKR and 5 PFU of VV IκBα M per cell; 4, 5 PFU of VV and 5 PFU of VV IκBα M per cell; 5, 3 PFU of VV PKR and 7 PFU of hBcl2 per dell; 6, 3 PFU of VV PKR and 7 PFU of VV IκBα M per cell; 7, 3 PFU of VV PKR and 7 PFU of VV per cell; 8, 3 PFU of VV PKR and 7 PFU of VV aBcl-2 per cell. (B) Immunoblot analysis of PKR and IκBα M expression with the anti-SV5 tag antibody in the same extracts used to measure apoptosis. HeLa cells (C), PKR0/0 (D), or PKR+/+ (E) cells grown in 12-well plates were infected at an MOI of 10 with the viruses indicated in the presence of 5 mM IPTG and harvested at 24 hpi for the determination of apoptosis by using the cell death detection ELISA. Columns: 1, 3 PFU of VV PKR and 7 PFU of VV per cell; 2, 3 PFU of VV PKR and 7 PFU of VV IκBα M per cell; 3, 3 PFU of VV PKR and 7 PFU of VV IκBα S per cell; 4, 10 PFU of VV per cell. Triplicate experiments were performed.
FIG. 7
Coinfection of VV PKR with VV IκBα M inhibits PKR-induced apoptosis. (A) BSC-40 cells grown in 12-well plates were infected at an MOI of 10 with the viruses indicated in the presence of 5 mM IPTG and harvested at 24 hpi to determine the absorbance at 405 nm. The mean values of triplicate experiments with standard deviations are given. Columns: 1, 5 PFU of VV PKR and 5 PFU of VV per cell; 2, 5 PFU of VV PKR and 5 PFU of VV aBcl-2 per cell; 3, 5 PFU of VV PKR and 5 PFU of VV IκBα M per cell; 4, 5 PFU of VV and 5 PFU of VV IκBα M per cell; 5, 3 PFU of VV PKR and 7 PFU of hBcl2 per dell; 6, 3 PFU of VV PKR and 7 PFU of VV IκBα M per cell; 7, 3 PFU of VV PKR and 7 PFU of VV per cell; 8, 3 PFU of VV PKR and 7 PFU of VV aBcl-2 per cell. (B) Immunoblot analysis of PKR and IκBα M expression with the anti-SV5 tag antibody in the same extracts used to measure apoptosis. HeLa cells (C), PKR0/0 (D), or PKR+/+ (E) cells grown in 12-well plates were infected at an MOI of 10 with the viruses indicated in the presence of 5 mM IPTG and harvested at 24 hpi for the determination of apoptosis by using the cell death detection ELISA. Columns: 1, 3 PFU of VV PKR and 7 PFU of VV per cell; 2, 3 PFU of VV PKR and 7 PFU of VV IκBα M per cell; 3, 3 PFU of VV PKR and 7 PFU of VV IκBα S per cell; 4, 10 PFU of VV per cell. Triplicate experiments were performed.
FIG. 7
Coinfection of VV PKR with VV IκBα M inhibits PKR-induced apoptosis. (A) BSC-40 cells grown in 12-well plates were infected at an MOI of 10 with the viruses indicated in the presence of 5 mM IPTG and harvested at 24 hpi to determine the absorbance at 405 nm. The mean values of triplicate experiments with standard deviations are given. Columns: 1, 5 PFU of VV PKR and 5 PFU of VV per cell; 2, 5 PFU of VV PKR and 5 PFU of VV aBcl-2 per cell; 3, 5 PFU of VV PKR and 5 PFU of VV IκBα M per cell; 4, 5 PFU of VV and 5 PFU of VV IκBα M per cell; 5, 3 PFU of VV PKR and 7 PFU of hBcl2 per dell; 6, 3 PFU of VV PKR and 7 PFU of VV IκBα M per cell; 7, 3 PFU of VV PKR and 7 PFU of VV per cell; 8, 3 PFU of VV PKR and 7 PFU of VV aBcl-2 per cell. (B) Immunoblot analysis of PKR and IκBα M expression with the anti-SV5 tag antibody in the same extracts used to measure apoptosis. HeLa cells (C), PKR0/0 (D), or PKR+/+ (E) cells grown in 12-well plates were infected at an MOI of 10 with the viruses indicated in the presence of 5 mM IPTG and harvested at 24 hpi for the determination of apoptosis by using the cell death detection ELISA. Columns: 1, 3 PFU of VV PKR and 7 PFU of VV per cell; 2, 3 PFU of VV PKR and 7 PFU of VV IκBα M per cell; 3, 3 PFU of VV PKR and 7 PFU of VV IκBα S per cell; 4, 10 PFU of VV per cell. Triplicate experiments were performed.
FIG. 8
Coexpression of increasing amounts of IκBα Wt or M equally inhibit induction of apoptosis triggered by PKR. (A) BSC-40 cells grown in 12-well plates were infected at an MOI of 6 with the viruses indicated in the presence of 5 mM IPTG and harvested at 24 hpi for the determination of the absorbance at 405 nm. Mean values of triplicate experiments with standard deviations are given. Columns: 1, 2 PFU of VV PKR and 4 PFU of VV per cell; 2, 2 PFU of VV PKR, 2 PFU of VV IκBα M, and 2 PFU of VV per cell; 3, 2 PFU of VV PKR and 4 PFU of VV IκBα M per cell; 4, 4 PFU of VV IκBα M and 2 PFU of VV per cell; 5, 2 PFU of VV PKR, 2 PFU of VV IκBα Wt, and 2 PFU of VV per cell; 6, 2 PFU of VV PKR and 4 PFU of VV IκBα Wt per cell; 7, 4 PFU of VV IκBα Wt and 2 PFU of VV per cell. (B) Immunoblot analysis of PKR and IκBα M and Wt expression with the anti-SV5 tag antibody from the same extracts used to measure apoptosis.
FIG. 9
Coexpression of IκBα M with PKR inhibits NF-κB activity without affecting translational abrogation. (A) HeLa cells grown in 100-mm-diameter plates were mock infected or infected with the viruses indicated in the presence of 5 mM IPTG. When noted, 20 or 60 μM PSI was added to cells after 1 h of viral adsorption. Cells were collected at 20 hpi, and nuclear extracts were prepared and analyzed by gel shift assay as described above. Lanes: 1, 8 PFU of VV per cell; 2 to 4, 4 PFU of VV and 4 PFU of VV PKR per cell in the absence of PSI (lane 2) or with 20 or 60 μM (lanes 3 and 4, respectively); 5, 4 PFU of VV PKR and 4 PFU of VV IκBα M per cell; 6, 4 PFU of VV PKR and 4 PFU of VV IκBα WT per cell; 7, mock infected. (B) BSC-40 cells grown in 12-well plates were infected at an MOI of 6 with the viruses indicated in the presence of 5 mM IPTG, transfected 1 h later with 0.5 μg of pPR15, and harvested at 24 hpi; half of the samples were lysed in buffer for luciferase determination as described in Materials and Methods. Mean values of relative luciferase units (n = 3) measured in equal extracts, along with the standard deviations, are given. Columns: 1, 2 PFU of VV PKR and 4 PFU of VV per cell; 2, 2 PFU of VV PKR and 4 PFU of VV aBcl-2 per cell; 3, 2 PFU of VV PKR and 4 PFU of VV IκBα M per cell; 4, 4 PFU of VV IκBα M and 2 PFU of VV per cell. (C) The other half of the samples were processed according to the manufacturer’s instructions, and the absorbance at 405 nm was determined as a measure of apoptosis. Mean values of triplicate experiments with standard deviations are given. (D) BSC-40 cells were infected with the indicated viruses at the indicated concentrations, and the [35S]Met-Cys incorporation during a 1-h pulse was measured beginning at 12 hpi as described in Materials and Methods. Specific activities (in counts per minute per microgram of protein) are represented as the mean value from two experiments, along with the standard deviation.
Similar articles
- Protein kinase PKR amplification of interferon β induction occurs through initiation factor eIF-2α-mediated translational control.
McAllister CS, Taghavi N, Samuel CE. McAllister CS, et al. J Biol Chem. 2012 Oct 19;287(43):36384-92. doi: 10.1074/jbc.M112.390039. Epub 2012 Sep 4. J Biol Chem. 2012. PMID: 22948139 Free PMC article. - The catalytic activity of dsRNA-dependent protein kinase, PKR, is required for NF-kappaB activation.
Gil J, Rullas J, García MA, Alcamí J, Esteban M. Gil J, et al. Oncogene. 2001 Jan 18;20(3):385-94. doi: 10.1038/sj.onc.1204109. Oncogene. 2001. PMID: 11313968 - Okadaic acid induces apoptosis through double-stranded RNA-dependent protein kinase/eukaryotic initiation factor-2alpha pathway in human osteoblastic MG63 cells.
Morimoto H, Okamura H, Yoshida K, Kitamura S, Haneji T. Morimoto H, et al. J Biochem. 2004 Oct;136(4):433-8. doi: 10.1093/jb/mvh144. J Biochem. 2004. PMID: 15625311 - Double-stranded RNA-dependent protein kinase (PKR) in antiviral defence in fish and mammals.
Chaumont L, Collet B, Boudinot P. Chaumont L, et al. Dev Comp Immunol. 2023 Aug;145:104732. doi: 10.1016/j.dci.2023.104732. Epub 2023 May 10. Dev Comp Immunol. 2023. PMID: 37172664 Review. - The dsRNA protein kinase PKR: virus and cell control.
García MA, Meurs EF, Esteban M. García MA, et al. Biochimie. 2007 Jun-Jul;89(6-7):799-811. doi: 10.1016/j.biochi.2007.03.001. Epub 2007 Mar 12. Biochimie. 2007. PMID: 17451862 Review.
Cited by
- PANoptosis-related molecular subtype and prognostic model associated with the immune microenvironment and individualized therapy in pancreatic cancer.
Zhang B, Huang B, Zhang X, Li S, Zhu J, Chen X, Song H, Shang D. Zhang B, et al. Front Oncol. 2023 Jul 14;13:1217654. doi: 10.3389/fonc.2023.1217654. eCollection 2023. Front Oncol. 2023. PMID: 37519797 Free PMC article. - Formation of Double Stranded RNA Provokes Smooth Muscle Contractions and Structural Modifications in Bladder Ischemia.
Yang JH, Zhao Z, Niu W, Choi HP, Azadzoi KM. Yang JH, et al. Res Rep Urol. 2022 Nov 16;14:399-414. doi: 10.2147/RRU.S388464. eCollection 2022. Res Rep Urol. 2022. PMID: 36415310 Free PMC article. - GZ17-6.02 kills prostate cancer cells in vitro and in vivo.
Booth L, Roberts JL, West C, Dent P. Booth L, et al. Front Oncol. 2022 Nov 3;12:1045459. doi: 10.3389/fonc.2022.1045459. eCollection 2022. Front Oncol. 2022. PMID: 36408163 Free PMC article. - Immunogenicity of _In Vitro_-Transcribed RNA.
Mu X, Hur S. Mu X, et al. Acc Chem Res. 2021 Nov 2;54(21):4012-4023. doi: 10.1021/acs.accounts.1c00521. Epub 2021 Oct 22. Acc Chem Res. 2021. PMID: 34677064 Free PMC article. Review. - Protein Kinase R in Bacterial Infections: Friend or Foe?
Smyth R, Sun J. Smyth R, et al. Front Immunol. 2021 Jul 8;12:702142. doi: 10.3389/fimmu.2021.702142. eCollection 2021. Front Immunol. 2021. PMID: 34305942 Free PMC article. Review.
References
- Abbaddie C N, Kabrun F, Bouali F, Vandenbunder B, Enrietto P. High levels of c-rel expression are associated with programmed cell death in the developing avian embryo and in bone marrow cells in vitro. Cell. 1993;75:899–912. - PubMed
- Alcamí J, Lain de Lera T, Folgueira L, Pedraza M A, Jacqué J M, Bachelerie F, Noriega A R, Hay R T, Harrich D, Gaynor R B, Virelizier J L, Arenzana-Seisdedos F. Absolute dependence on κB responsive elements for initiation and Tat-mediated amplification of HIV transcription in blood CD4 T lymphocytes. EMBO J. 1995;14:1552–1560. - PMC - PubMed
- Baeuerle P A, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous