SDF-2 induction of terminal differentiation in Dictyostelium discoideum is mediated by the membrane-spanning sensor kinase DhkA - PubMed (original) (raw)
SDF-2 induction of terminal differentiation in Dictyostelium discoideum is mediated by the membrane-spanning sensor kinase DhkA
N Wang et al. Mol Cell Biol. 1999 Jul.
Abstract
SDF-2 is a peptide released by prestalk cells during culmination that stimulates prespore cells to encapsulate. Genetic evidence indicates that the response is dependent on the dhkA gene. This gene encodes a member of the histidine kinase family of genes that functions in two-component signal transduction pathways. The sequence of the N-terminal half of DhkA predicts two hydrophobic domains separated by a 310-amino-acid loop that could bind a ligand. By inserting MYC6 epitopes into DhkA, we were able to show that the loop is extracellular while the catalytic domain is cytoplasmic. Cells expressing the MYC epitope in the extracellular domain of DhkA were found to respond only if induced with 100-fold-higher levels of SDF-2 than required to induce dhkA+ cells; however, they could be induced to sporulate by addition of antibodies specific to the MYC epitope. To examine the enzymatic activity of DhkA, we purified the catalytic domain following expression in bacteria and observed incorporation of labelled phosphate from ATP consistent with histidine autophosphorylation. Site-directed mutagenesis of histidine1395 to glutamine in the catalytic domain blocked autophosphorylation. Furthermore, genetic analyses showed that histidine1395 and the relay aspartate2075 of DhkA are both critical to its function but that another histidine kinase, DhkB, can partially compensate for the lack of DhkA activity. Sporulation is drastically reduced in double mutants lacking both DhkA and DhkB. Suppressor studies indicate that the cyclic AMP (cAMP) phosphodiesterase RegA and the cAMP-dependent protein kinase PKA act downstream of DhkA.
Figures
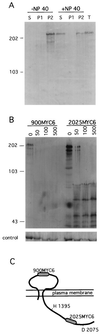
FIG. 1
Membrane association and orientation of the DhkA protein. (A) Cells expressing the 900MYC6 epitope-tagged DhkA protein were disrupted in the presence or absence of 0.5% NP-40, as indicated. The supernatant (S) and pelleted material (P) following high-speed centrifugation, as well as unfractionated extract (T), were resolved by gel electrophoresis and analyzed by Western blotting with a monoclonal antibody against the MYC epitope. Each sample contains material from 2 × 107 cells. The sizes and positions of protein markers are indicated on the left, in kilodaltons. (B) Intact cells expressing the 900MYC6 epitope or the 2025MYC6 epitope were disaggregated by trituration in isotonic buffer and treated with 0 to 500 μg of proteinase K per ml, as indicated above the respective lanes. Cells were washed free of the protease and resuspended in sample buffer. Samples representing 2 × 107 cells each were resolved by gel electrophoresis and analyzed by Western blotting with a monoclonal antibody against the MYC epitope. Numbers on the left indicate sizes, in kilodaltons. As a control, aliquots from the same samples were analyzed with antibodies against the intracellular protein TipA. (C) Schematic representation of the DhkA protein (2150 amino acids) relative to the plasma membrane. The MYC6 epitopes are represented by shaded areas. H 1393 and D 2075 represent the conserved histidine residue in the H motif and the conserved aspartic acid residue in the D motif, respectively. The drawing is not to scale.
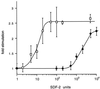
FIG. 2
Reduced sensitivity to SDF-2 in dhkA900MYC6 cells. Wild-type cells of strain AX4 (□) and _dhkA_− cells carrying the DhkA 900MYC6 construct (●) were developed on filters until early culmination, dissociated, and deposited as a monolayer under buffer. Purified SDF-2 was added at various concentrations, and spores were counted after 6 h. Fold stimulation was calculated relative to the number of spores seen in cultures incubated in the absence of added SDF-2. As a control, we added 50 units of SDF-1 and found that sporulation was stimulated 2.3 ± 0.2-fold for both wild-type dhkA+ cells and dhkA900MYC6 cells.
FIG. 3
Terminal differentiation of the _dhkA− dhkB_− double mutant. Wild-type (AX4) cells (left) and a _dhkB_− derivative of strain AK299 (_dhkA_−) (right) were developed for 36 h on filter supports. The fruiting bodies of the double-mutant (_dhkA− dhkB_−) strain had long weak stalks that often toppled over before they could be photographed. Prespore cells of the double mutant ascended the stalks but never encapsulated to form spores.
FIG. 4
Autophosphorylation of DhkA. Proteins from an equal number of bacteria carrying p_dhkAcat_ (A), p_dhkAcatH1395Q_ (B), or vector alone (C) were bound to metal affinity beads and incubated with [32P]ATP. Proteins were eluted, resolved by gel electrophoresis, and exposed to X-ray film. (D) Proteins were then transferred to a nitrocellulose filter that was treated with 1 M HCl for 2 h, and the portion shown in lane A was exposed for the same period of time to X-ray film. The arrow indicates the position of the 70-kDa protein.
Similar articles
- Signal transduction pathways leading to spore differentiation in Dictyostelium discoideum.
Anjard C, Zeng C, Loomis WF, Nellen W. Anjard C, et al. Dev Biol. 1998 Jan 15;193(2):146-55. doi: 10.1006/dbio.1997.8804. Dev Biol. 1998. PMID: 9473320 - Genetic interactions of the E3 ubiquitin ligase component FbxA with cyclic AMP metabolism and a histidine kinase signaling pathway during Dictyostelium discoideum development.
Tekinay T, Ennis HL, Wu MY, Nelson M, Kessin RH, Ratner DI. Tekinay T, et al. Eukaryot Cell. 2003 Jun;2(3):618-26. doi: 10.1128/EC.2.3.618-626.2003. Eukaryot Cell. 2003. PMID: 12796307 Free PMC article. - Cytokinins induce sporulation in Dictyostelium.
Anjard C, Loomis WF. Anjard C, et al. Development. 2008 Mar;135(5):819-27. doi: 10.1242/dev.018051. Epub 2008 Jan 23. Development. 2008. PMID: 18216168 - Histidine kinases in signal transduction pathways of eukaryotes.
Loomis WF, Shaulsky G, Wang N. Loomis WF, et al. J Cell Sci. 1997 May;110 ( Pt 10):1141-5. doi: 10.1242/jcs.110.10.1141. J Cell Sci. 1997. PMID: 9191038 Review. - Role of PKA in the timing of developmental events in Dictyostelium cells.
Loomis WF. Loomis WF. Microbiol Mol Biol Rev. 1998 Sep;62(3):684-94. doi: 10.1128/MMBR.62.3.684-694.1998. Microbiol Mol Biol Rev. 1998. PMID: 9729606 Free PMC article. Review.
Cited by
- Evolutionary proteomics identifies amino acids essential for ligand-binding of the cytokinin receptor CHASE domain.
Heyl A, Wulfetange K, Pils B, Nielsen N, Romanov GA, Schmülling T. Heyl A, et al. BMC Evol Biol. 2007 Apr 17;7:62. doi: 10.1186/1471-2148-7-62. BMC Evol Biol. 2007. PMID: 17439640 Free PMC article. - Evolution of multicellularity in Dictyostelia.
Kawabe Y, Du Q, Schilde C, Schaap P. Kawabe Y, et al. Int J Dev Biol. 2019;63(8-9-10):359-369. doi: 10.1387/ijdb.190108ps. Int J Dev Biol. 2019. PMID: 31840775 Free PMC article. Review. - Evolutionary crossroads in developmental biology: Dictyostelium discoideum.
Schaap P. Schaap P. Development. 2011 Feb;138(3):387-96. doi: 10.1242/dev.048934. Development. 2011. PMID: 21205784 Free PMC article. Review. - Outside-in signaling of cellulose synthesis by a spore coat protein in Dictyostelium.
West CM, Zhang P, McGlynn AC, Kaplan L. West CM, et al. Eukaryot Cell. 2002 Apr;1(2):281-92. doi: 10.1128/EC.1.2.281-292.2002. Eukaryot Cell. 2002. PMID: 12455962 Free PMC article. - From environmental sensing to developmental control: cognitive evolution in dictyostelid social amoebas.
Schaap P. Schaap P. Philos Trans R Soc Lond B Biol Sci. 2021 Mar 15;376(1820):20190756. doi: 10.1098/rstb.2019.0756. Epub 2021 Jan 25. Philos Trans R Soc Lond B Biol Sci. 2021. PMID: 33487113 Free PMC article.
References
- Abe H, Uchiyama M, Tanaka Y, Saito H. Structure of discadenine, a spore germination inhibitor from the cellular slime mold Dictyostelium discoideum. Tetrahedron Lett. 1976;42:3807–3810.
- Abe K, Yanagisawa K. A new class of rapid developing mutants in Dictyostelium discoideum: implications for cyclic AMP metabolism and cell differentiation. Dev Biol. 1983;95:200–210. - PubMed
- Alex L, Simon M. Protein histidine kinases and signal transduction in prokaryotes and eukaryotes. Trends Genet. 1994;10:133–138. - PubMed
- Anjard C, Pinaud S, Kay R R, Reymond C D. Overexpression of DdPK2 protein kinase causes rapid development and affects the intracellular cAMP pathway of Dictyostelium discoideum. Development. 1992;115:785–790. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials