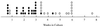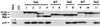p53 mediates apoptotic crisis in primary Abelson virus-transformed pre-B cells - PubMed (original) (raw)
p53 mediates apoptotic crisis in primary Abelson virus-transformed pre-B cells
I Unnikrishnan et al. Mol Cell Biol. 1999 Jul.
Abstract
Transformation of pre-B cells by Abelson murine leukemia virus (Ab-MLV) involves a balance between positive, growth-stimulatory signals from the v-Abl oncoprotein and negative regulatory cues from cellular genes. This phenomenon is reflected by the clonal selection that occurs during Ab-MLV-mediated transformation in vivo and in vitro. About 50% of all Ab-MLV-transformed pre-B cells express mutant forms of p53 as they emerge from this process, suggesting that this protein may play an important role in the transformation process. Consistent with this idea, expression of p19(Arf), a protein whose function depends on the presence of a functional p53, is required for the apoptotic crisis that characterizes primary Ab-MLV transformants. To test the role of p53 in pre-B-cell transformation directly, we examined the response of Trp53(-/-) mice to Ab-MLV. The absence of p53 shortens the latency of Abelson disease induction but does not affect the frequency of cells susceptible to Ab-MLV-induced transformation. However, primary transformants derived from the null animals bypass the apoptotic crisis that characterizes the transition from primary transformant to fully malignant cell line. These effects do not require p21(Cip-1), a major downstream target of p53; however, consistent with a role of p19(Arf), transformants expressing mutant p53 and abundant p19 retain wild-type p19 sequences.
Figures
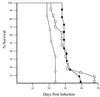
FIG. 1
Accelerated tumor induction in _Trp53_−/− mice. Age-matched _Trp53_−/− (○), Trp53+/− (□), and Trp53+/+ (●) mice were injected with Ab-MLV-P160 and monitored for tumor development. Animals were sacrificed when tumors were evident; each point represents a single mouse.
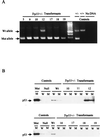
FIG. 2
Trp53+/− transformants lose their remaining Trp53 allele rapidly. (A) DNAs from representative Trp53+/− transformants were amplified with primers specific for the wild-type and targeted alleles, and products were fractionated through an agarose gel containing ethidium bromide. The numbers above each lane identify the cell clone from which the sample was derived. DNAs from Trp53+/− and Trp53+/+ mice and a reaction mix containing no DNA were used as controls. The unmarked lane contains a 100-bp DNA ladder, used as a marker. Arrows denote positions of the wild-type (Wt) and mutant (Mut) specific PCR products. (B) Lysates from the cell lines analyzed in panel A and control cell lines were immunoprecipitated with anti-p53 antibody Ab-4, specific for wild-type p53 (lanes W), or anti-p53 antibody Ab-3, specific for mutant forms of p53, (lanes M) and the immunoprecipitates were analyzed by Western blotting with anti-p53 antibody Ab-7, which recognizes both mutant and wild-type p53 forms on Western blots. The p53 statuses of the control wild-type (204-3-1), mutant (143-2M), and null (L1-2) cell lines were characterized previously (47).
FIG. 3
Trp53 is required for crisis induction. Primary transformants from Trp53+/+ (●) and _Trp53_−/− (□) mice were assessed for viability by using trypan blue staining when they were removed from agar cultures and at regular intervals thereafter.
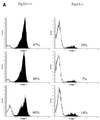
FIG. 4
Trp53+/+ transformants undergo apoptotic crisis during outgrowth. (A) Three independent Trp53+/+ and _Trp53_−/− transformants were stained with MC540 (31) and analyzed by flow cytometry. The percentages of apoptotic cells, represented by black peaks, are noted. The data shown are representative of analyses of more than 10 additional independent transformants from each background. (B) DNA was prepared as described in Materials and Methods from representative Trp53+/+ and _Trp53_−/− transformants and fractionated through an agarose gel containing ethidium bromide. The data shown are representative of analyses of 10 independent transformants from each background. Lane M, 100-bp ladder marker.
FIG. 4
Trp53+/+ transformants undergo apoptotic crisis during outgrowth. (A) Three independent Trp53+/+ and _Trp53_−/− transformants were stained with MC540 (31) and analyzed by flow cytometry. The percentages of apoptotic cells, represented by black peaks, are noted. The data shown are representative of analyses of more than 10 additional independent transformants from each background. (B) DNA was prepared as described in Materials and Methods from representative Trp53+/+ and _Trp53_−/− transformants and fractionated through an agarose gel containing ethidium bromide. The data shown are representative of analyses of 10 independent transformants from each background. Lane M, 100-bp ladder marker.
FIG. 5
_p21Cip-1_−/− transformants undergo crisis. Growth and viability of primary transformants from _p21Cip-1_−/− mice were monitored as described in Materials and Methods. The times at which primary transformants succumbed to crisis (●) and at which cell lines that survived crisis and became established (○) are indicated.
FIG. 6
p19Arf is expressed in transformants from Trp53 null mice. Lysates were prepared from transformants from Trp53 null animals or wild-type (WT) mice and examined by Western blotting for the presence of the p19Arf protein. The cells used were derived with either the P120, P90, or P80 strains of Ab-MLV (24). The transformation properties of these viruses are similar in wild-type and Trp53 null mice (our unpublished data). Lysates from NIH 3T3 (p19Arf-negative) cells and from the p19Arf-positive cell line MEL (29) were used as controls. The blots were also probed with the H548 anti-Gag/v-Abl monoclonal antibody (7) to control for protein loading.
Similar articles
- p19(Arf) induces p53-dependent apoptosis during abelson virus-mediated pre-B cell transformation.
Radfar A, Unnikrishnan I, Lee HW, DePinho RA, Rosenberg N. Radfar A, et al. Proc Natl Acad Sci U S A. 1998 Oct 27;95(22):13194-9. doi: 10.1073/pnas.95.22.13194. Proc Natl Acad Sci U S A. 1998. PMID: 9789064 Free PMC article. - Changes in p19Arf localization accompany apoptotic crisis during pre-B-cell transformation by Abelson murine leukemia virus.
Zimmerman RS, Rosenberg N. Zimmerman RS, et al. J Virol. 2008 Sep;82(17):8383-91. doi: 10.1128/JVI.00348-08. Epub 2008 Jun 25. J Virol. 2008. PMID: 18579612 Free PMC article. - p16(Ink4a) interferes with Abelson virus transformation by enhancing apoptosis.
Sachs Z, Sharpless NE, DePinho RA, Rosenberg N. Sachs Z, et al. J Virol. 2004 Apr;78(7):3304-11. doi: 10.1128/jvi.78.7.3304-3311.2004. J Virol. 2004. PMID: 15016851 Free PMC article. - Loss of heterozygosity at the Ink4a/Arf locus facilitates Abelson virus transformation of pre-B cells.
Mostecki J, Halgren A, Radfar A, Sachs Z, Ravitz J, Thome KC, Rosenberg N. Mostecki J, et al. J Virol. 2000 Oct;74(20):9479-87. doi: 10.1128/jvi.74.20.9479-9487.2000. J Virol. 2000. PMID: 11000217 Free PMC article. - Absence of p53 complements defects in Abelson murine leukemia virus signaling.
Unnikrishnan I, Rosenberg N. Unnikrishnan I, et al. J Virol. 2003 Jun;77(11):6208-15. doi: 10.1128/jvi.77.11.6208-6215.2003. J Virol. 2003. PMID: 12743277 Free PMC article.
Cited by
- Decreased virus population diversity in p53-null mice infected with weakly oncogenic Abelson virus.
Marchlik E, Kalman R, Rosenberg N. Marchlik E, et al. J Virol. 2005 Sep;79(18):11618-26. doi: 10.1128/JVI.79.18.11618-11626.2005. J Virol. 2005. PMID: 16140739 Free PMC article. - p53 deficiency increases transformation by v-Abl and rescues the ability of a C-terminally truncated v-Abl mutant to induce pre-B lymphoma in vivo.
Zou X, Cong F, Coutts M, Cattoretti G, Goff SP, Calame K. Zou X, et al. Mol Cell Biol. 2000 Jan;20(2):628-33. doi: 10.1128/MCB.20.2.628-633.2000. Mol Cell Biol. 2000. PMID: 10611241 Free PMC article. - Tumor-intrinsic sensitivity to the pro-apoptotic effects of IFN-γ is a major determinant of CD4+ CAR T-cell antitumor activity.
Boulch M, Cazaux M, Cuffel A, Guerin MV, Garcia Z, Alonso R, Lemaître F, Beer A, Corre B, Menger L, Grandjean CL, Morin F, Thieblemont C, Caillat-Zucman S, Bousso P. Boulch M, et al. Nat Cancer. 2023 Jul;4(7):968-983. doi: 10.1038/s43018-023-00570-7. Epub 2023 May 29. Nat Cancer. 2023. PMID: 37248395 Free PMC article. - Analysis of p53 inactivation in a human T-cell leukemia virus type 1 Tax transgenic mouse model.
Portis T, Grossman WJ, Harding JC, Hess JL, Ratner L. Portis T, et al. J Virol. 2001 Mar;75(5):2185-93. doi: 10.1128/JVI.75.5.2185-2193.2001. J Virol. 2001. PMID: 11160722 Free PMC article. - Rapid, stabilizing palindrome rearrangements in somatic cells by the center-break mechanism.
Cunningham LA, Coté AG, Cam-Ozdemir C, Lewis SM. Cunningham LA, et al. Mol Cell Biol. 2003 Dec;23(23):8740-50. doi: 10.1128/MCB.23.23.8740-8750.2003. Mol Cell Biol. 2003. PMID: 14612414 Free PMC article.
References
- Baker S, Markowitz S, Fearson E, Wilson J, Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990;249:912–914. - PubMed
- Blyth K, Terry A, O’Hara M, Baxter E W, Campbell M, Stewart M, Donehower L A, Onions D E, Neil J C, Cameron E R. Synergy between a human c-myc transgene and p53 null genotype in murine thymic lymphomas: contrasting effects of homozygous and heterozygous p53 loss. Oncogene. 1995;10:1717–1723. - PubMed
- Brugarolas J, Chandrasekaran C, Gordon J I, Beach D, Jacks T, Hannon G J. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature (London) 1995;377:552–556. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous