T-Cell responses during Trypanosoma brucei infections in mice deficient in inducible nitric oxide synthase - PubMed (original) (raw)
T-Cell responses during Trypanosoma brucei infections in mice deficient in inducible nitric oxide synthase
A E Millar et al. Infect Immun. 1999 Jul.
Abstract
We have investigated the possibility that nitric oxide (NO) synthesis may affect the course of a trypanosome infection via T-cell responses using mice deficient in inducible NO synthase (iNOS). Parasitemia levels increased at the same rate in both iNOS-deficient homozygous and control heterozygous mice, and peak parasitemia values were the same in both groups. However, the heterozygous mice maintained higher parasitemia levels after the peak of an infection than the homozygous mice due to a decrease in the rate of clearance of parasites. In iNOS-deficient mice there was an increase in the numbers of total CD4(+) cells and activated (interleukin-2 receptor-expressing) CD4(+) cells in infected mice compared with the numbers in uninfected mice. Spleen cells from infected iNOS-deficient mice displayed increased proliferative responses and gamma interferon secretion when stimulated in vitro than those of control mice. These data suggest that NO production depresses T-helper 1-like responses generated during Trypanosoma brucei infections, thus promoting the survival of the parasite.
Figures
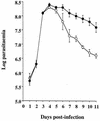
FIG. 1
Time course of T. brucei parasitemia in iNOS-deficient (○) and heterozygous control (●) mice inoculated with 104 GUTat 7.2 parasites. Results are expressed as geometric means ±2 SE for groups of five mice.
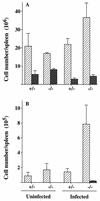
FIG. 2
Numbers of CD4+ (light) or CD8+ (dark) cells per spleen (A) and numbers of activated CD4+ (light) or CD8+ (dark) cells per spleen, with CD25 as a marker for cell activation (B). Numbers were determined by flow cytometry of mononuclear splenocytes of iNOS-deficient (−/−) and control (+/−) mice, either uninfected or infected with GUTat 7.2 (results are for day 11 of infection). Mononuclear splenocytes were counted, the lymphocyte population was gated and checked to exclude autofluorescence, and relative numbers were determined. From these data, cell numbers per spleen were calculated and are expressed as geometric means ±2 SE for groups of five mice.
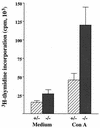
FIG. 3
Proliferative responses of mononuclear splenocytes from trypanosome-infected iNOS-deficient (−/−) (dark) and control (+/−) (light) mice harboring infections of GUTat 7.2. Splenocytes were cultured in the presence of either medium or ConA (8 μg/ml). Proliferative responses of mononuclear splenocytes from uninfected mice were <2,000 cpm in the absence of stimulation. Results are expressed as the geometric means ±2 SE for groups of five mice.
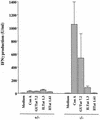
FIG. 4
IFN-γ production by mononuclear splenocytes from iNOS-deficient mice and their heterozygous counterparts harboring infections with GUTat 7.2. IFN-γ concentrations were assayed by enzyme-linked immunosorbent assay. Cells were stimulated with medium, ConA (8 μg/ml), or paraformaldehyde-fixed trypanosomes (2 × 106/ml) expressing the homologous VAT or one of the two heterologous VATs. Supernatants were harvested 72 h after stimulation. Results are expressed as means ±2 SE for groups of five mice.
Similar articles
- Concurrent infections with Trypanosoma brucei and Nippostrongylus brasiliensis in mice deficient in inducible nitric oxide.
Chiejina S, Goyal P, Li C, Wakelin D. Chiejina S, et al. Parasitol Int. 2003 Jun;52(2):107-15. doi: 10.1016/s1383-5769(02)00089-2. Parasitol Int. 2003. PMID: 12798922 - IFN-gamma-dependent nitric oxide production is not linked to resistance in experimental African trypanosomiasis.
Hertz CJ, Mansfield JM. Hertz CJ, et al. Cell Immunol. 1999 Feb 25;192(1):24-32. doi: 10.1006/cimm.1998.1429. Cell Immunol. 1999. PMID: 10066343 - Mouse strain susceptibility to trypanosome infection: an arginase-dependent effect.
Duleu S, Vincendeau P, Courtois P, Semballa S, Lagroye I, Daulouède S, Boucher JL, Wilson KT, Veyret B, Gobert AP. Duleu S, et al. J Immunol. 2004 May 15;172(10):6298-303. doi: 10.4049/jimmunol.172.10.6298. J Immunol. 2004. PMID: 15128819 - Alternative versus classical macrophage activation during experimental African trypanosomosis.
Baetselier PD, Namangala B, Noël W, Brys L, Pays E, Beschin A. Baetselier PD, et al. Int J Parasitol. 2001 May 1;31(5-6):575-87. doi: 10.1016/s0020-7519(01)00170-9. Int J Parasitol. 2001. PMID: 11334945 Review. - Analysis of macrophage activation in African trypanosomiasis.
Paulnock DM, Coller SP. Paulnock DM, et al. J Leukoc Biol. 2001 May;69(5):685-90. J Leukoc Biol. 2001. PMID: 11358974 Review.
Cited by
- Developmental regulation and extracellular release of a VSG expression-site-associated gene product from Trypanosoma brucei bloodstream forms.
Barnwell EM, van Deursen FJ, Jeacock L, Smith KA, Maizels RM, Acosta-Serrano A, Matthews K. Barnwell EM, et al. J Cell Sci. 2010 Oct 1;123(Pt 19):3401-11. doi: 10.1242/jcs.068684. Epub 2010 Sep 7. J Cell Sci. 2010. PMID: 20826456 Free PMC article. - Role for inducible nitric oxide synthase in protection from chronic Chlamydia trachomatis urogenital disease in mice and its regulation by oxygen free radicals.
Ramsey KH, Sigar IM, Rana SV, Gupta J, Holland SM, Byrne GI. Ramsey KH, et al. Infect Immun. 2001 Dec;69(12):7374-9. doi: 10.1128/IAI.69.12.7374-7379.2001. Infect Immun. 2001. PMID: 11705910 Free PMC article. - Inducible nitric oxide synthase regulates production of isoprostanes in vivo during chlamydial genital infection in mice.
Ramsey KH, Sigar IM, Rana SV, Gupta J, Holland SM, Byrne GI, Morrow JD. Ramsey KH, et al. Infect Immun. 2003 Dec;71(12):7183-7. doi: 10.1128/IAI.71.12.7183-7187.2003. Infect Immun. 2003. PMID: 14638813 Free PMC article. - Interferon gamma eliminates responding CD4 T cells during mycobacterial infection by inducing apoptosis of activated CD4 T cells.
Dalton DK, Haynes L, Chu CQ, Swain SL, Wittmer S. Dalton DK, et al. J Exp Med. 2000 Jul 3;192(1):117-22. doi: 10.1084/jem.192.1.117. J Exp Med. 2000. PMID: 10880532 Free PMC article. - Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. II. Effects on microbial proliferation and host survival in vivo.
Mastroeni P, Vazquez-Torres A, Fang FC, Xu Y, Khan S, Hormaeche CE, Dougan G. Mastroeni P, et al. J Exp Med. 2000 Jul 17;192(2):237-48. doi: 10.1084/jem.192.2.237. J Exp Med. 2000. PMID: 10899910 Free PMC article.
References
- Askonas B A. Macrophages as mediators of immunosuppression in murine African trypanosomiasis. Curr Top Microbiol Immunol. 1985;117:119–127. - PubMed
- Borowy N K, Sternberg J M, Schreiber D, Nonnengasser C, Overath P. Suppressive macrophages occurring in murine Trypanosoma brucei infection inhibit T-cell responses in vivo and in vitro. Parasite Immunol. 1990;12:233–246. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials