Developmental requirement of gp130 signaling in neuronal survival and astrocyte differentiation - PubMed (original) (raw)
Developmental requirement of gp130 signaling in neuronal survival and astrocyte differentiation
K Nakashima et al. J Neurosci. 1999.
Abstract
gp130 is a signal-transducing receptor component used in common by the interleukin-6 (IL-6) family of hematopoietic and neurotrophic cytokines, including IL-6, IL-11, leukemia-inhibitory factor, ciliary neurotrophic factor, oncostatin-M, and cardiotrophin-1. We have examined in this study a role of gp130 in the nervous system by analyzing developmental cell death of several neuronal populations and the differentiation of astrocytes in gp130-deficient mice. A significant reduction was observed in the number of sensory neurons in L5 dorsal root ganglia and motoneurons in the facial nucleus, the nucleus ambiguus, and the lumbar spinal cord in gp130 -/- mice on embryonic day 18.5. On the other hand, no significant neuronal loss was detectable on day 14.5, suggesting a physiological role of gp130 in supporting newly generated neurons during the late phase of development when naturally occurring cell death takes place. Moreover, expression of an astrocyte marker, GFAP, was severely reduced in the brain of gp130 -/- mice. Our data demonstrate that gp130 expression is essential for survival of subgroups of differentiated motor and sensory neurons and for the differentiation of major populations of astrocytes in vivo.
Figures
Fig. 1.
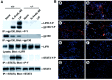
Impairment of astrocyte induction in neuroepithelial cells by the lack of gp130. A, Absence of gp130 signalings in gp130-deficient cells. Neuroepithelial cells from E14.5 gp130 +/+ and gp130 −/− mice were stimulated with either IL-6 plus sIL-6R or LIF. NP-40 lysates were subjected to immunoprecipitation and subsequent immunoblotting with the antibodies indicated, except for the middle panel in which the lysates were directly analyzed by immunoblotting. B, Loss of astrocyte induction in gp130-deficient cells in vitro. Neuroepithelial cells from E14.5 gp130 +/+ (a–d) or gp130 −/− (e–h) mice were cultured as described in Materials and Methods with medium alone (a, e), or IL-6 plus sIL-6R (b, f), LIF (c,g), or CNTF (d, h) and stained for GFAP by specific antibody. GFAP-positive cells and nuclei are shown in red and blue, respectively, by fluorescent microscopy.
Fig. 2.
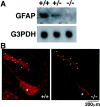
Impairment of GFAP expression in gp130-deficient brain. A, Loss of GFAP transcripts in gp130-deficient brain. RNA from E18.5 gp130 +/+, +/−, and −/− brain was analyzed by Northern blotting with a GFAP-specific probe. B, Reduction of GFAP-positive cells in gp130-deficient brain. Cryosections from E18.5 gp130 +/+ and gp130 −/− brain were stained with GFAP-specific antibody and rhodamine-conjugated second antibody.Asterisks indicate fimbria. The dotted outlines represent the molecular layer of dentate gyrus. No detectable staining was observed when the first antibody was omitted during the procedures (data not shown).
Fig. 3.
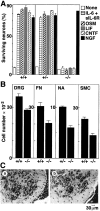
Neuronal defects in gp130-deficient mice.A, Complete loss of survival response of DRG neurons to gp130 stimulation. DRG neurons from E18.5 mice with the indicated genotype were cultured for 20 hr with gp130-stimulating cytokines, as well as NGF. Live neurons were counted under phase-contrast microscopy.B, Reduction of neuron numbers in gp130-deficient mice. Histological sections prepared from E18.5 mice with the indicated genotype were used for counting neurons in L5 DRG (p < 0.05; t test), facial nucleus (FN) (p < 0.05), nucleus ambiguus (NA), and L1–L6 spinal motor column (SMC) (p < 0.005). Each column represents mean ± SD of the data from three individual mice, except for gp130 +/− nucleus ambiguus in which the mean ± SD fluctuation of the data from two mice was indicated. C, Histological view of spinal motor neurons. Representative sections from gp130 +/+ (a) and gp130 −/− (b) spinal ventral horn are shown.
Similar articles
- Astrocyte differentiation of fetal neuroepithelial cells by interleukin-11 via activation of a common cytokine signal transducer, gp130, and a transcription factor, STAT3.
Yanagisawa M, Nakashima K, Arakawa H, Ikenaka K, Yoshida K, Kishimoto T, Hisatsune T, Taga T. Yanagisawa M, et al. J Neurochem. 2000 Apr;74(4):1498-504. doi: 10.1046/j.1471-4159.2000.0741498.x. J Neurochem. 2000. PMID: 10737606 - Cardiotrophin-1, a muscle-derived cytokine, is required for the survival of subpopulations of developing motoneurons.
Oppenheim RW, Wiese S, Prevette D, Armanini M, Wang S, Houenou LJ, Holtmann B, Gotz R, Pennica D, Sendtner M. Oppenheim RW, et al. J Neurosci. 2001 Feb 15;21(4):1283-91. doi: 10.1523/JNEUROSCI.21-04-01283.2001. J Neurosci. 2001. PMID: 11160399 Free PMC article. - Astrocyte gp130 expression is critical for the control of Toxoplasma encephalitis.
Drögemüller K, Helmuth U, Brunn A, Sakowicz-Burkiewicz M, Gutmann DH, Mueller W, Deckert M, Schlüter D. Drögemüller K, et al. J Immunol. 2008 Aug 15;181(4):2683-93. doi: 10.4049/jimmunol.181.4.2683. J Immunol. 2008. PMID: 18684959 - Gp130, a shared signal transducing receptor component for hematopoietic and neuropoietic cytokines.
Taga T. Taga T. J Neurochem. 1996 Jul;67(1):1-10. doi: 10.1046/j.1471-4159.1996.67010001.x. J Neurochem. 1996. PMID: 8666978 Review. - Gp130 and the interleukin-6 family of cytokines.
Taga T, Kishimoto T. Taga T, et al. Annu Rev Immunol. 1997;15:797-819. doi: 10.1146/annurev.immunol.15.1.797. Annu Rev Immunol. 1997. PMID: 9143707 Review.
Cited by
- Enhanced gene activation by Notch and BMP signaling cross-talk.
Takizawa T, Ochiai W, Nakashima K, Taga T. Takizawa T, et al. Nucleic Acids Res. 2003 Oct 1;31(19):5723-31. doi: 10.1093/nar/gkg778. Nucleic Acids Res. 2003. PMID: 14500836 Free PMC article. - Histone acetylation-mediated glycosyltransferase gene regulation in mouse brain during development.
Suzuki Y, Yanagisawa M, Ariga T, Yu RK. Suzuki Y, et al. J Neurochem. 2011 Mar;116(5):874-80. doi: 10.1111/j.1471-4159.2010.07042.x. Epub 2011 Jan 7. J Neurochem. 2011. PMID: 21214566 Free PMC article. - DLK1 promotes neurogenesis of human and mouse pluripotent stem cell-derived neural progenitors via modulating Notch and BMP signalling.
Surmacz B, Noisa P, Risner-Janiczek JR, Hui K, Ungless M, Cui W, Li M. Surmacz B, et al. Stem Cell Rev Rep. 2012 Jun;8(2):459-71. doi: 10.1007/s12015-011-9298-7. Stem Cell Rev Rep. 2012. PMID: 21761283 - MET Receptor Tyrosine Kinase Regulates Lifespan Ultrasonic Vocalization and Vagal Motor Neuron Development.
Kamitakahara AK, Ali Marandi Ghoddousi R, Lanjewar AL, Magalong VM, Wu HH, Levitt P. Kamitakahara AK, et al. Front Neurosci. 2021 Nov 4;15:768577. doi: 10.3389/fnins.2021.768577. eCollection 2021. Front Neurosci. 2021. PMID: 34803597 Free PMC article. - The Role of BMP Signaling in Endothelial Heterogeneity.
Han O, Pak B, Jin SW. Han O, et al. Front Cell Dev Biol. 2021 Jun 21;9:673396. doi: 10.3389/fcell.2021.673396. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34235147 Free PMC article. Review.
References
- Barres BA, Burne JF, Holtmann B, Thoenen H, Sendtner M, Raff MC. Ciliary neurotrophic factor enhances the rate of oligodendrocyte generation. Mol Cell Neurosci. 1996;8:146–156. - PubMed
- Betz UAK, Bloch W, van den Broeck M, Yoshida K, Taga T, Zinkernagel R, Kishimoto T, Addicks K, Rajewsky K, Müller WJ. Postnatally induced inactivation of gp130 in mice results in neurological, cardiac, hematopoietic, immunological, hepatic and pulmonary defects. J Exp Med. 1998;188:1955–1965. - PMC - PubMed
- Bonni A, Sun Y, Nadal-Vicens M, Bhatt A, Frank DA, Rozovsky I, Stahl N, Yancopoulos GD, Greenberg ME. Regulation of gliogenesis in the central nervous system by the JAK–STAT signaling pathway. Science. 1997;278:477–483. - PubMed
- Davis S, Aldrich TH, Stahl N, Pan L, Taga T, Kishimoto T, Ip NY, Yancopoulos GD. LIFRβ and gp130 as heterodimerizing signal transducers of the tripartite CNTF receptor. Science. 1993;260:1805–1808. - PubMed
- DeChiara TM, Vejsada R, Poueymirou WT, Acheson A, McClain J, Pan L, Stahl N, Ip NY, Kato A, Yancopoulos GD. Mice lacking the CNTF receptor, unlike mice lacking CNTF, exhibit profound motor neuron deficits at birth. Cell. 1995;83:313–322. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous