Localization of a suprachiasmatic nucleus subregion regulating locomotor rhythmicity - PubMed (original) (raw)
Localization of a suprachiasmatic nucleus subregion regulating locomotor rhythmicity
J LeSauter et al. J Neurosci. 1999.
Abstract
The bilaterally symmetrical suprachiasmatic nuclei (SCN) of the hypothalamus are the loci of the mammalian clock controlling circadian rhythms. Previous studies suggested that all regions of the SCN are equipotential as circadian rhythmicity is sustained after partial ablation, as long as approximately 25% of the nuclei are spared. In contrast to these results, we found that animals bearing partial lesions of the SCN that spared the subregion delimited by cells containing the calcium-binding protein calbindin-D28K (CaBP), sustained circadian locomotor rhythms. Furthermore, there was a correlation between the strength of the rhythm and the number of spared CaBP cells. Partial lesions that destroyed this region but spared other compartments of the SCN resulted in loss of rhythmicity. The next study indicates that transplants of half-SCN grafts that contain CaBP cells restore locomotor rhythms in SCN-lesioned host animals, whereas transplants containing SCN tissue but lacking cells of this subnucleus fail to restore rhythmicity. Finally, there was a correlation between the number of CaBP-positive cells in the graft and the strength of the restored rhythm. Taken together, the results indicate that pacemakers in the region of the CaBP subnucleus are necessary and sufficient for the control of locomotor rhythmicity and that the SCN is functionally heterogeneous.
Figures
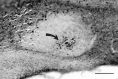
Fig. 1.
Photomicrograph of a sagittal brain section showing the location of the CaBP subnucleus (arrow). This subregion is highly localized in the caudal SCN, making it possible to ablate this part of the nucleus but to leave other compartments intact. Scale bar, 200 μm.
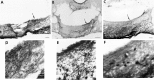
Fig. 2.
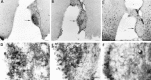
Photomicrographs of coronal brain sections showing a partial SCN lesion in animal B26-V34. Much of the SCN is spared in this animal, although circadian rhythmicity is lost after the lesion (behavior shown in Fig. 4_A_).A_–_C, Adjacent low-power sections stained for NP, VIP, and CaBP, respectively. A. Low-power view of a section through the SCN stained for NP (arrows_indicate NP cells in the dorsomedial SCN). B. The lateral aspect of the SCN is damaged (asterisks). The_arrow points to the region shown in high power in_E_. C, No immunostained cells could be detected in the SCN, although sparse CaBP cells can be seen in the adjacent hypothalamus (arrow). The _box_denotes the region that is shown in high power in F.D_–_F, High-power photomicrographs of sections stained for NP, VIP, and CaBP, respectively. No CaBP cells could be detected in the SCN. Scale bars:A_–_C, 100 μm; D,E, 10 μm; F, 20 μm.
Fig. 3.
Photomicrographs of coronal brain sections showing a partial SCN lesion in animal B56-CA29 (behavior shown in Fig.4_B_). A_–_C, Adjacent low-power sections stained for NP, VIP, and CaBP, respectively.A, B, Asterisks indicates partial SCN ablation at the ventral aspect. The arrows_in A and B point to the region shown in high power in D and E, respectively.C. No CaBP cells could be detected in the SCN. The_box denotes the region that is shown in high power in_F_. D–F, High-power photomicrographs. The animal lost locomotor rhythmicity (Fig. 4_B_) after the lesion, although NP (D) and VIP (E) cells (arrows) and fibers were spared. F, No CaBP cells could be detected in the SCN. Scale bars: A–C, 200 μm; D, F, 20 μm; E, 10 μm.
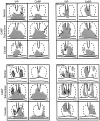
Fig. 4.
Wheel running rhythm of the animals for which anatomy are presented in Figures 2 and 3. To facilitate inspection of the rhythms, the daily activity is plotted twice on a 48 hr time scale. The animals received an SCN lesion (SCN-X) at the point indicated on the_left_ of the actogram. Spectral analyses of the data are shown on the right of the actograms. The black vertical bars on the right of the actograms indicate the days (intact and lesioned) for which the analyses were done. A, The intact heterozygote tau mutant hamster B26-V34 had a period of 22.6 hr (days 1–12). After the partial SCN lesion, it became arrhythmic (analyses shown for days 34–74).B, The intact wild-type hamster B56-CA29 had a period of 24 hr (days 1–12). The animal became arrhythmic after a partial lesion of the SCN ablating the CaBP subnucleus (analysis shown for days 42–67).
Fig. 5.
Schematics of the caudal aspect of the SCN depicting the area of damage (gray) in hamsters with partial SCN lesions in one animal with sparing and three animals with ablation of the CaBP subregion. The approximate outline of the SCN is indicated by broken lines. For each animal, the drawings show sections stained for VIP and CaBP. For each peptide, the schematics are shown at three levels, separated by 200 μm through areas rostral to, centered in, and caudal to the CaBP subnucleus. Animal B54-CA23 (top left panel) had a few CaBP cells remaining on one side (arrow). This animal was rhythmic after the lesion. For the other three animals, much of the SCN is spared, and VIP cells and fibers were seen, but CaBP-IR cells were not detected. These three animals were arrhythmic after the lesion.
Fig. 6.
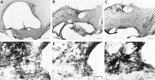
Photomicrographs of coronal brain sections showing the transplant containing the SCN in animal B28-Q66 (behavior shown in Fig. 8_A_). A, The graft lies caudal to the lesion site. There is a plexus of NP staining (arrow) indicating the presence of the donor SCN.B, In a section 50 μm from A, VIP fibers (arrow) overlap the region of the NP fiber plexus; the graft borders are indicated by a dashed line. C, Section adjacent to _B_showing CaBP cells at the same level as the NP and VIP fibers.D_–_F, Higher magnifications of the areas marked by arrows in A_–_C, respectively. Scale bars: A_–_C, 200 μm;D_–_F, 20 μm.
Fig. 7.
Photomicrographs of coronal brain sections showing the transplant containing the SCN in animal B33-Q67 (behavior shown in Fig. 8_B_). A, The graft (borders are indicated by a dashed line) lies caudal to the lesion site. There is a plexus of NP staining (arrow) indicating the presence of the donor SCN. B, In a section 50 μm from A, no VIP plexus was seen within the graft. C, Section adjacent to _B_showing a cluster of CaBP cells at the same level as the NP plexus.D_–_F, Higher magnifications of the areas marked by arrows in A_–_C, respectively. D, Plexus of NP fibers within the graft.E, Absence of VIP staining. F, CaBP subnucleus within the graft, surrounded by a space devoid of CaBP cells (asterisks). Scale bars: A–C, 200 μm;D–F, 20 μm.
Fig. 8.
Wheel running rhythm of heterozygote tau mutants for which the anatomical data are presented in Figures 6 and 7. The animals received an SCN lesion (SCN-X) and received a half-SCN transplant at the points indicated on the_left_ of the actogram (see Fig. 4 legend for further details). A, The intact hamster B28-Q66 had a free-running period of 21.9 hr (days 1–8) and became arrhythmic after an SCN lesion (days 12–22). After transplantation, it recovered with the donor period of 24.8 hr (days 43–71). B, The intact hamster B33-Q67 had a period of 21.9 hr (days 1–8). After an SCN lesion, it became arrhythmic (days 12–22). After transplantation, it recovered with the donor period of 24.0 hr (days 61–103).
Fig. 9.
Photomicrographs of coronal brain sections of the SCN within the grafts in animal B46–28T (behavior shown in Fig.11_A_). A, The graft (borders are indicated by a dashed line) lies caudal to the lesion site. There is a plexus of NP staining (arrow) indicating the presence of the donor SCN. B, In a section 50 μm from A, a plexus of VIP fibers (arrow) lies at the same level as the NP plexus.C, The graft lacks CaBP cells, although many CaBP cells can be seen in the host’s hypothalamus.D_–_F, Higher magnifications of the areas marked by arrows in A_–_C, respectively. D, E, Plexus of NP and VIP cells (arrows) and fibers within the graft (open arrows indicate nonspecific immunoreactivity within the ventricular epithelium in E). F, No CaBP cells can be detected within the brain parenchyma at the level of the NP and VIP plexi (open arrows indicate nonspecific immunoreactivity within the ventricular epithelium). Scale bars:A_–_C, 200 μm;D_–_F, 20 μm.
Fig. 10.
Photomicrographs of coronal brain sections of the SCN within the grafts in animal B39-Q70 (behavior shown in Fig.11_B_). A, The graft (borders are indicated by a dashed line) lies caudal to the lesion site. A plexus of NP staining (arrow) indicates the location of the donor SCN. B, In a section 50 μm from_A_, there are a few VIP cells and fibers (arrow) at the same level as the NP plexus.C, The SCN region lacks the cluster of CaBP cells, although sparse CaBP cells can be seen in other parts of the graft.D_–_F, Higher magnifications of the areas marked by arrows in A_–_C, respectively. D, E, Plexus of NP and VIP cells (arrows) and fibers within the graft.F, Although there is some nonspecific staining around the edges of ventricles and blood vessels in the area where NP and VIP are present, no CaBP cells could be detected in the SCN. Scale bars: A, 200 μm; B, C, 100 μm; D_–_F, 20 μm.
Fig. 11.
Wheel running rhythm of the heterozygotes that did not recover rhythmicity after transplantation (anatomical data are presented in Figs. 9, 10). The animals were lesioned (SCN-X) and received a half-SCN transplant at the points indicated on the left of the actogram (details of legend and analysis shown in Fig. 4). A, The intact hamster B46–28T had a free-running period of 22.6 hr (days 1–10). After an SCN lesion, it became arrhythmic (days 19–41).B, Intact animal B39-Q70 had a free-running period of 22.6 hr (days 1–7). It became arrhythmic (days 10–23) after SCN ablation.
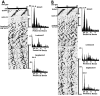
Fig. 12.
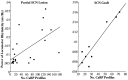
There is a positive correlation between the number of SCN CaBP profiles and the absolute power of the rhythm in animals with a partial lesion of the SCN (A;r = 0.61; p = 0.002) and in the lesioned-grafted animals (B; r = 0.94; p = 0.0002).
Similar articles
- Calbindin expression in the hamster SCN is influenced by circadian genotype and by photic conditions.
LeSauter J, Stevens P, Jansen H, Lehman MN, Silver R. LeSauter J, et al. Neuroreport. 1999 Oct 19;10(15):3159-63. doi: 10.1097/00001756-199910190-00007. Neuroreport. 1999. PMID: 10574553 - Attachment site of grafted SCN influences precision of restored circadian rhythm.
LeSauter J, Romero P, Cascio M, Silver R. LeSauter J, et al. J Biol Rhythms. 1997 Aug;12(4):327-38. doi: 10.1177/074873049701200405. J Biol Rhythms. 1997. PMID: 9438881 - Targeted microlesions reveal novel organization of the hamster suprachiasmatic nucleus.
Kriegsfeld LJ, LeSauter J, Silver R. Kriegsfeld LJ, et al. J Neurosci. 2004 Mar 10;24(10):2449-57. doi: 10.1523/JNEUROSCI.5323-03.2004. J Neurosci. 2004. PMID: 15014120 Free PMC article. - Cellular requirements of suprachiasmatic nucleus transplants for restoration of circadian rhythm.
Boer GJ, van Esseveldt LE, Rietveld WJ. Boer GJ, et al. Chronobiol Int. 1998 Sep;15(5):551-66. doi: 10.3109/07420529808998707. Chronobiol Int. 1998. PMID: 9787941 Review. - Output signals of the SCN.
LeSauter J, Silver R. LeSauter J, et al. Chronobiol Int. 1998 Sep;15(5):535-50. doi: 10.3109/07420529808998706. Chronobiol Int. 1998. PMID: 9787940 Review.
Cited by
- Intracellular calcium spikes in rat suprachiasmatic nucleus neurons induced by BAPTA-based calcium dyes.
Hong JH, Min CH, Jeong B, Kojiya T, Morioka E, Nagai T, Ikeda M, Lee KJ. Hong JH, et al. PLoS One. 2010 Mar 10;5(3):e9634. doi: 10.1371/journal.pone.0009634. PLoS One. 2010. PMID: 20224788 Free PMC article. - Socially synchronized circadian oscillators.
Bloch G, Herzog ED, Levine JD, Schwartz WJ. Bloch G, et al. Proc Biol Sci. 2013 Jul 3;280(1765):20130035. doi: 10.1098/rspb.2013.0035. Print 2013 Aug 22. Proc Biol Sci. 2013. PMID: 23825203 Free PMC article. - Serotonergic integration of circadian clock and ultradian sleep-wake cycles.
Miyamoto H, Nakamaru-Ogiso E, Hamada K, Hensch TK. Miyamoto H, et al. J Neurosci. 2012 Oct 17;32(42):14794-803. doi: 10.1523/JNEUROSCI.0793-12.2012. J Neurosci. 2012. PMID: 23077063 Free PMC article. - Dose-dependent effects of androgens on the circadian timing system and its response to light.
Butler MP, Karatsoreos IN, LeSauter J, Silver R. Butler MP, et al. Endocrinology. 2012 May;153(5):2344-52. doi: 10.1210/en.2011-1842. Epub 2012 Apr 4. Endocrinology. 2012. PMID: 22492303 Free PMC article. - Circadian trafficking of calbindin-ir in fibers of SCN neurons.
LeSauter J, Bhuiyan T, Shimazoe T, Silver R. LeSauter J, et al. J Biol Rhythms. 2009 Dec;24(6):488-96. doi: 10.1177/0748730409350876. J Biol Rhythms. 2009. PMID: 19926808 Free PMC article.
References
- Aguilar-Roblero R, Morin LP, Moore RY. Morphological correlates of circadian rhythm restoration induced by transplantation of the suprachiasmatic nucleus in hamsters. Exp Neurol. 1994;130:250–260. - PubMed
- Buijs RM, Hou YX, Shin S, Renaud LP. Ultrastructural evidence for intra- and extranuclear projections of GABA-ergic neurons of the suprachiasmatic nucleus. J Comp Neurol. 1994;340:381–391. - PubMed
- Bryant DN, LeSauter J, Silver R, Romero M-T. Retinal synapses on calbindin-ir cells of the hamster suprachiasmatic nucleus. Soc Neurosc Abstr. 1996;22:1140.
- Coggeshall RE, Lekan HA. Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J Comp Neurol. 1996;364:6–15. - PubMed
- Davis FC, Gorski RA. Development of hamster circadian rhythms: role of the maternal suprachiasmatic nucleus. J Comp Physiol. 1988;162:601–610. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous