Reprogramming of intestinal differentiation and intercalary regeneration in Cdx2 mutant mice - PubMed (original) (raw)
Reprogramming of intestinal differentiation and intercalary regeneration in Cdx2 mutant mice
F Beck et al. Proc Natl Acad Sci U S A. 1999.
Abstract
The homeobox gene Cdx2, a homologue of the Drosophila gene caudal, has been implicated in the control of cell differentiation in the intestinal epithelium. Recently, we showed that mice in which one allele of the Cdx2 gene had been inactivated by homologous recombination developed multiple intestinal polyp-like lesions that did not express Cdx2 and that contained areas of squamous metaplasia in the form of keratinizing stratified squamous epithelium, similar to that occurring in the mouse esophagus and forestomach. We have now examined colonic lesions from 98 Cdx2+/- mice and report that the lesions are composed of heterotopic stomach and small intestinal mucosa. We conclude that Cdx2 directs endodermal differentiation toward a caudal phenotype and that haploinsufficient levels of expression in the developing distal intestine lead to homeotic transformation to a more rostral endodermal phenotype, such as forestomach epithelium that does not express Cdx2 during normal development. Intercalary growth (epimorphic regeneration), which previously has never been described in mammals, then occurs, resulting in the ordered "filling in" of tissue types at the discontinuity between the gastric and colonic epithelia. This intercalary growth in a restricted space results in the formation of the polypoid lesions observed.
Figures
Figure 1
The frequency of polyps in 98 mice plotted against their age. The numbers of animals at each monthly time point are indicated in brackets. The slope of the line is not significantly different from zero (P = 0.92).
Figure 2
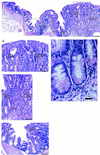
Portion of a colonic polyp from a Cdx2+/− mouse showing the sequence SSE, gastric cardia (GCa) containing columnar mucus-secreting cells with basal nuclei, and gastric corpus (GCo) containing clearly identifiable parietal (oxyntic) cells (arrows). (Bar = 45 μm.)
Figure 3
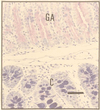
Montage showing the lateral edge of a colonic polyp in a Cdx2+/− mouse. (Top) The sequence—gastric corpus (GCo), gastric antrum (GA), small intestine (SI), and colon (C)—is clearly seen. (Bar = 130 μm.) (A) Transition from GCo to GA. Parietal (oxyntic) cells (closed arrow heads) and enterochromaffin-like cells (arrows) are easily identifiable, as are the columnar mucus-secreting cells of the gastric antrum (open arrow heads). (Bar = 55 μm.) (B) Transition from GA to SI. Goblet cells in the villi and Paneth cells (arrow) situated in the crypts are clearly seen adjacent to the branched mucus-secreting cells of the gastric antrum. (Bar = 55 μm.) (C) Transition from SI to C. Stunted villi with goblet cells and Paneth cells (arrow) in the crypts between villi, lie adjacent to colonic tissue. (Bar = 55 μm.) (D) Portion of B magnified further to show Paneth cells with characteristic eosinophilic granules. (Bar = 20 μm.)
Figure 4
Portion of a colonic polyp showing the junction of gastric cardia (GCa) and gastric corpus (GCo). (Upper) This section is stained with hematoxylin and eosin. (Lower) An adjacent section was incubated with antiserum to the gastric proton pump to locate oxyntic cells in the gastric corpus. (Bars = 55 μm.)
Figure 5
This section of a colonic polyp shows characteristics of gastric antrum (GA) and normal colon (C) distal to it. The section was stained by Mowry’s technique (13) to show PAS-staining (neutral) mucopolysaccharides in the polyp (upper portion of section) and alcian-blue-staining (acid) mucopolysaccharides in the normal colon (lower portion of section). (Bar = 50 μm.)
Figure 6
A section adjacent to Fig. 5 was stained for TFF2. The heterotopic tissue with the histological phenotype of gastric antrum (upper portion of section) stains strongly, whereas the phenotypically normal colonic-type mucosa is negative (lower portion of section). (Bar = 50 μm.)
Figure 7
Ectopic SSE in the small intestine of a 5-day-old Cdx2+/− mouse. The large endosomal vacuoles in the villous enterocytes (arrow) are typical of the small intestine before weaning. (Bar = 80 μm.)
Figure 8
Polyp-like lesion in the proximal colon of a Cdx2+/− mouse. The cells in the main part of the lesion stain negatively with Cdx2 antibody and are typical of the mucous glands of pyloric antrum. The intercalated small intestinal-type mucosa at the edge of the lesion, however, stains positive for Cdx2 (arrowhead). (Bar = 100 μm.)
Similar articles
- Cdx2 ectopic expression induces gastric intestinal metaplasia in transgenic mice.
Silberg DG, Sullivan J, Kang E, Swain GP, Moffett J, Sund NJ, Sackett SD, Kaestner KH. Silberg DG, et al. Gastroenterology. 2002 Mar;122(3):689-96. doi: 10.1053/gast.2002.31902. Gastroenterology. 2002. PMID: 11875002 - Expression of Cdx1 and Cdx2 mRNAs and relevance of this expression to differentiation in human gastrointestinal mucosa--with special emphasis on participation in intestinal metaplasia of the human stomach.
Mizoshita T, Inada K, Tsukamoto T, Kodera Y, Yamamura Y, Hirai T, Kato T, Joh T, Itoh M, Tatematsu M. Mizoshita T, et al. Gastric Cancer. 2001;4(4):185-91. doi: 10.1007/pl00011741. Gastric Cancer. 2001. PMID: 11846061 - CDX2 regulates liver intestine-cadherin expression in normal and malignant colon epithelium and intestinal metaplasia.
Hinoi T, Lucas PC, Kuick R, Hanash S, Cho KR, Fearon ER. Hinoi T, et al. Gastroenterology. 2002 Nov;123(5):1565-77. doi: 10.1053/gast.2002.36598. Gastroenterology. 2002. PMID: 12404231 - Homeosis and polyposis: a tale from the mouse.
He TC, da Costa LT, Thiagalingam S. He TC, et al. Bioessays. 1997 Jul;19(7):551-5. doi: 10.1002/bies.950190705. Bioessays. 1997. PMID: 9230687 Review. - The role of CDX2 in inflammatory bowel disease.
Coskun M. Coskun M. Dan Med J. 2014 Mar;61(3):B4820. Dan Med J. 2014. PMID: 24814920 Review.
Cited by
- MISP Is Overexpressed in Intestinal Metaplasia and Gastric Cancer.
Vilarinho T, Pádua D, Pereira B, Mesquita P, Almeida R. Vilarinho T, et al. Curr Oncol. 2024 May 14;31(5):2769-2779. doi: 10.3390/curroncol31050210. Curr Oncol. 2024. PMID: 38785491 Free PMC article. - Tissue-location-specific transcription programs drive tumor dependencies in colon cancer.
Yang L, Tu L, Bisht S, Mao Y, Petkovich D, Thursby SJ, Liang J, Patel N, Yen RC, Largent T, Zahnow C, Brock M, Gabrielson K, Salimian KJ, Baylin SB, Easwaran H. Yang L, et al. Nat Commun. 2024 Feb 15;15(1):1384. doi: 10.1038/s41467-024-45605-4. Nat Commun. 2024. PMID: 38360902 Free PMC article. - Gastric heterotopia of colon found cancer workup in liver abscess: A case report.
Park JG, Suh JI, Kim YU. Park JG, et al. World J Clin Cases. 2022 May 26;10(15):5012-5017. doi: 10.12998/wjcc.v10.i15.5012. World J Clin Cases. 2022. PMID: 35801043 Free PMC article. - CDX2-induced intestinal metaplasia in human gastric organoids derived from induced pluripotent stem cells.
Koide T, Koyanagi-Aoi M, Uehara K, Kakeji Y, Aoi T. Koide T, et al. iScience. 2022 Apr 28;25(5):104314. doi: 10.1016/j.isci.2022.104314. eCollection 2022 May 20. iScience. 2022. PMID: 35602937 Free PMC article. - Transcription factor CDX2 up-regulates proto-oncogenic miR-744 via a promoter activation mechanism in non-small-cell lung cancer.
Sha Z, Hao S, Long F, Wei Y, Chen S, Li T, Yi L, Hu L, Lin Z, Xian J, Pei X. Sha Z, et al. Ann Transl Med. 2021 Oct;9(20):1538. doi: 10.21037/atm-21-4526. Ann Transl Med. 2021. PMID: 34790744 Free PMC article.
References
- Brooke N M, Garcia-Fernandez J, Holland P W. Nature (London) 1998;392:920–922. - PubMed
- Mlodzik M, Gehring W. Cell. 1987;48:465–478. - PubMed
- Burglin T R. In: Guidebook to the Homeobox Genes. Duboule D, editor. New York: Oxford Univ. Press; 1994. pp. 27–71.
- Duprey P, Chowdhury K, Dressler G R, Balling R, Simon D, Guernet J L, Gruss P. Genes Dev. 1988;2:1647–1654. - PubMed
- Meyer B L, Gruss P. Development. 1993;117:191–203. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials