Selective activation of the versican promoter by epithelial- mesenchymal interactions during hair follicle development - PubMed (original) (raw)
Selective activation of the versican promoter by epithelial- mesenchymal interactions during hair follicle development
J Kishimoto et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Interaction between the epithelium and the mesenchyme is an essential feature of organogenesis, including hair follicle formation. The dermal papilla (DP), a dense aggregate of specialized dermis-derived stromal cells located at the bottom of the follicle, is a major component of hair that signals the follicular epithelial cells to prolong the hair growth process. However, little is known about DP-specific gene activation with regard to hair induction. In this study we demonstrate that a short fragment (839 bp) of the human versican (a core protein of one of the matrix chondroitin sulfate proteoglycans) promoter is sufficient to activate lacZ reporter gene expression in the DP of postnatal transgenic mice and also in the condensed mesenchyme (the origin of the DP) beneath the hair placode during hair follicle embryogenesis. Using the same versican promoter with green fluorescent protein (GFP), large numbers of fresh pelage DP cells were isolated from newborn transgenic skin by high-speed cell sorting. These GFP-positive DP cells showed abundant versican mRNA, confirming that the reporter molecules reflected endogenous versican gene expression. These sorted GFP-positive cells showed DP-like morphology in culture, but both GFP and versican expression was lost during primary culture. In vivo hair growth assays showed that GFP-positive cells could induce hair when grafted with epithelial cells, whereas GFP-negative cells grafted with epithelium or GFP-positive cells alone did not. These results suggest that versican may play an essential role both in mesenchymal condensation and in hair induction.
Figures
Figure 1
Construction of versican–lacZ transgene. The number in parentheses indicates the human versican sequence position from the major transcription site as +1 (arrow). Synthetic splice donor and acceptor sites are labeled SD/SA; polyadenylation signal sequence is labeled pA. ●, _Eco_RI; ○, _Xho_I; rectangular box, _Xba_I.
Figure 2
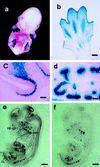
Analysis of versican–lacZ transgenic mouse embryos. (a) Whole-mount β-galactosidase staining of E13.5 embryo. (b) Paraffin section of E13.5 limb transgenic mouse after β-galactosidase staining. (c) LacZ in the mesenchyme adjacent to the olfactory epithelium at E14.5. (d) LacZ staining in the developing kidney at E14.5. (e and f) Mouse versican and lacZ mRNA expression in transgenic mice. (e) LacZ antisense probe. (f) Mouse versican antisense probe. OE, olfactory epithelium; HCL, humerus cartilage; KD, kidney; CL, cartilage. [Bar = 100 μm (b), 50 μm (c and d), and 500 μm (e and f).]
Figure 3
Versican–LacZ expression in hair follicle embryogenesis of transgenic mice. Paraffin sections of prestained embryonic skin for β-galactosidase activity. (a) Embryonic skin at E13.5. (b) Pelage hair germ at E14.5. (c) Pelage hair germ at E15.5. (d) Hair plug at E17.5. Arrowhead, condensed mesenchyme; dotted line, hair placode. [Bar = 50 μm (a and d) and 25 μm (b and c).]
Figure 4
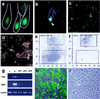
Versican–LacZ expression in postnatal skin of transgenic mice. (a) Newborn skin. LacZ staining in DP (arrowheads); faint LacZ in the upper dermis (asterisk). Mouse versican in situ hybridization (inner box); specific versican mRNA signal in DP (arrow). (b) Late-anagen hair follicle; all DP cells are still LacZ positive but the volume of DP decreases (arrowheads). (c) Catagen-to-telogen hair follicle—club hair shows no LacZ staining and faint LacZ activity in the second-germ DP (arrowhead). Asterisks show melanin pigmentation in the hair shaft. (d) Anagen hair follicle in the second hair cycle. Strong LacZ staining reappears in the DP (arrowhead). (Bar = 100 μm.)
Figure 5
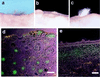
Isolation of fresh pelage dermal papilla from versican–GFP transgenic mouse. (a) Fluorescence microscopy of frozen section from newborn versican–GFP transgenic mouse skin. Line indicates hair follicle outline. (b) Partially dissociated dermal cell suspensions. GFP-positive DP at the bottom of undissociated follicles. (c) Complete dissociated cell suspension. DP-derived cells are now seen as single cells. (d) Undissociated follicle pellet removed from cell suspension. No DP associated with the remaining preformed follicle, indicating most of the DP cells were released into the cell suspension. (e_–_f) Cell-sorting chart of the dermal cells from versican–GFP transgenic mouse skin (e) and postsorting analysis of sorted GFP-positive cell pools (f). Upper windows indicate cells collected as GFP-positive and lower windows indicate those collected as GFP-negative cells. (g) RT-PCR analysis of sorted cells and subsequent culture mRNA expression. −, Negative sorted fresh cells; +, positive sorted fresh cells; +P1, +P2, +P3, passaged culture from GFP-positive sorted cells. Ver, mouse versican primers. (h and i) Cultured DP from positive sorted cells. (h) Primary culture day 1. (i) Primary culture day 4. (b_–_d, h, and i) Merged images from fluorescence and phase-contrast microscopy.
Figure 6
Hair-induction assay on nude mouse skin. (a) Grafting after 4 weeks with sorted GFP-positive cells + C3H epithelial cells. (b) Grafting with sorted negative cells + C3H epithelial cells. (c) Grafting after 4 months with sorted GFP-positive cells + CD-1 epithelial cells. (d and e) Frozen sections of the graft site after 3 weeks. (d) Grafting of sorted positive cells with epithelial cells. GFP fluorescence was seen at the condensed DP in the deeper dermis. (e) Grafting with sorted positive cells only. Note only diffused fluorescence was observed in the upper dermis and with no sign of condensation. (Bar = 100 μm.)
Similar articles
- Association of versican with dermal matrices and its potential role in hair follicle development and cycling.
du Cros DL, LeBaron RG, Couchman JR. du Cros DL, et al. J Invest Dermatol. 1995 Sep;105(3):426-31. doi: 10.1111/1523-1747.ep12321131. J Invest Dermatol. 1995. PMID: 7665924 - Cultured peribulbar dermal sheath cells can induce hair follicle development and contribute to the dermal sheath and dermal papilla.
McElwee KJ, Kissling S, Wenzel E, Huth A, Hoffmann R. McElwee KJ, et al. J Invest Dermatol. 2003 Dec;121(6):1267-75. doi: 10.1111/j.1523-1747.2003.12568.x. J Invest Dermatol. 2003. PMID: 14675169 - Versican gene: regulation by the β-catenin signaling pathway plays a significant role in dermal papilla cell aggregative growth.
Yang Y, Li Y, Wang Y, Wu J, Yang G, Yang T, Gao Y, Lu Y. Yang Y, et al. J Dermatol Sci. 2012 Dec;68(3):157-63. doi: 10.1016/j.jdermsci.2012.09.011. Epub 2012 Sep 28. J Dermatol Sci. 2012. PMID: 23099107 - Molecular control of epithelial-mesenchymal interactions during hair follicle cycling.
Botchkarev VA, Kishimoto J. Botchkarev VA, et al. J Investig Dermatol Symp Proc. 2003 Jun;8(1):46-55. doi: 10.1046/j.1523-1747.2003.12171.x. J Investig Dermatol Symp Proc. 2003. PMID: 12894994 Review. - Versican: signaling to transcriptional control pathways.
Rahmani M, Wong BW, Ang L, Cheung CC, Carthy JM, Walinski H, McManus BM. Rahmani M, et al. Can J Physiol Pharmacol. 2006 Jan;84(1):77-92. doi: 10.1139/y05-154. Can J Physiol Pharmacol. 2006. PMID: 16845893 Review.
Cited by
- Effects of Detergent-Based Protocols on Decellularization of Corneas With Sclerocorneal Limbus. Evaluation of Regional Differences.
González-Andrades M, Carriel V, Rivera-Izquierdo M, Garzón I, González-Andrades E, Medialdea S, Alaminos M, Campos A. González-Andrades M, et al. Transl Vis Sci Technol. 2015 Apr 10;4(2):13. doi: 10.1167/tvst.4.2.13. eCollection 2015 Apr. Transl Vis Sci Technol. 2015. PMID: 25909036 Free PMC article. - Fully functional hair follicle regeneration through the rearrangement of stem cells and their niches.
Toyoshima KE, Asakawa K, Ishibashi N, Toki H, Ogawa M, Hasegawa T, Irié T, Tachikawa T, Sato A, Takeda A, Tsuji T. Toyoshima KE, et al. Nat Commun. 2012 Apr 17;3:784. doi: 10.1038/ncomms1784. Nat Commun. 2012. PMID: 22510689 Free PMC article. - Increased expression of versican in the inflammatory response to UVB- and reactive oxygen species-induced skin tumorigenesis.
Kunisada M, Yogianti F, Sakumi K, Ono R, Nakabeppu Y, Nishigori C. Kunisada M, et al. Am J Pathol. 2011 Dec;179(6):3056-65. doi: 10.1016/j.ajpath.2011.08.042. Epub 2011 Oct 12. Am J Pathol. 2011. PMID: 22001346 Free PMC article. - Extremely Low-Frequency Electromagnetic Fields Increase the Expression of Anagen-Related Molecules in Human Dermal Papilla Cells via GSK-3β/ERK/Akt Signaling Pathway.
Ki GE, Kim YM, Lim HM, Lee EC, Choi YK, Seo YK. Ki GE, et al. Int J Mol Sci. 2020 Jan 25;21(3):784. doi: 10.3390/ijms21030784. Int J Mol Sci. 2020. PMID: 31991762 Free PMC article. - Dermal Papilla Cells: From Basic Research to Translational Applications.
Zhang HL, Qiu XX, Liao XH. Zhang HL, et al. Biology (Basel). 2024 Oct 20;13(10):842. doi: 10.3390/biology13100842. Biology (Basel). 2024. PMID: 39452150 Free PMC article. Review.
References
- Weinberg W C, Goodman L V, George C, Morgan D L, Ledbetter S, Yuspa S H, Lichti U. J Invest Dermatol. 1993;100:229–236. - PubMed
- Hardy M H. Trends Genet. 1992;8:55–61. - PubMed
- Messenger A G. J Invest Dermatol. 1993;101:4S–9S. - PubMed
- Oro A E, Scott M P. Cell. 1998;95:575–578. - PubMed
- du Cros D L, LeBaron R G, Couchman J R. J Invest Dermatol. 1995;105:426–431. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases