Properties of heterologously expressed hTRP3 channels in bovine pulmonary artery endothelial cells - PubMed (original) (raw)
Properties of heterologously expressed hTRP3 channels in bovine pulmonary artery endothelial cells
M Kamouchi et al. J Physiol. 1999.
Erratum in
- J Phusiol (Lond) 1999 Sep 15;519 Pt 3:923
Abstract
1. We combined patch clamp and fura-2 fluorescence methods to characterize human TRP3 (hTRP3) channels heterologously expressed in cultured bovine pulmonary artery endothelial (CPAE) cells, which do not express the bovine trp3 isoform (btrp3) but express btrp1 and btrp4. 2. ATP, bradykinin and intracellular InsP3 activated a non-selective cation current (IhTRP3) in htrp3-transfected CPAE cells but not in non-transfected wild-type cells. During agonist stimulation, the sustained rise in [Ca2+]i was significantly higher in htrp3-transfected cells than in control CPAE cells. 3. The permeability for monovalent cations was PNa > PCs approximately PK >> PNMDG and the ratio PCa/PNa was 1.62 +/- 0.27 (n = 11). Removal of extracellular Ca2+ enhanced the amplitude of the agonist-activated IhTRP3 as well as that of the basal current The trivalent cations La3+ and Gd3+ were potent blockers of IhTRP3 (the IC50 for La3+ was 24.4 +/- 0.7 microM). 4. The single-channel conductance of the channels activated by ATP, assessed by noise analysis, was 23 pS. 5. Thapsigargin and 2,5-di-tert-butyl-1, 4-benzohydroquinone (BHQ), inhibitors of the organellar Ca2+-ATPase, failed to activate IhTRP3. U-73122, a phospholipase C blocker, inhibited IhTRP3 that had been activated by ATP and bradykinin. Thimerosal, an InsP3 receptor-sensitizing compound, enhanced IhTRP3, but calmidazolium, a calmodulin antagonist, did not affect IhTRP3. 6. It is concluded that hTRP3 forms non-selective plasmalemmal cation channels that function as a pathway for agonist-induced Ca2+ influx.
Figures
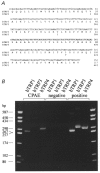
Figure 1. Nucleotide and deduced amino acid sequence of the bovine trp3 cDNA fragment aligned to the amino acid sequence of human trp3, and expression of bovine _trp_-related genes in CPAE cells
A, only those nucleotides outside the sequence of the degenerated primers used for amplification are shown. The numbering of htrp3 is according to Zhu et al. (1996). Identical amino acids are indicated by blanks. B, RT-PCR analysis using specific primer pairs, showing the expression of btrp1 and btrp4 but not_btrp3_ in CPAE cells. The left and right lanes show the DNA site markers in base pairs as indicated.
Figure 2. Effects of hTRP3 expression on the ATP-induced electrical response and changes in [Ca2+]i in CPAE cells
Comparison of the effects of ATP (1 μM) on membrane potential (top trace) and [Ca2+]i (bottom trace) in non-transfected (A) and _htrp3_-transfected CPAE cells (B). Membrane potential was measured using the current clamp mode. The I-V curves in C (non-transfected cells) and _D (htrp3_-transfected cells) measured before (a) and during application of ATP (b) were obtained from a voltage ramp protocol applied in voltage clamp mode at the correspondingly labelled points in A and B. For Ca2+ measurements the dotted line refers to 0 μM [Ca2+]i. Extracellular Ca2+ was 1·5 mM, except for the period marked by the horizontal bar in B (0 mM Ca2+).
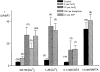
Figure 3. Effects of hTRP3 channels on agonist-stimulated Ca2+ transients
A and B, changes in [Ca2+]i as a result of ATP stimulation of a non-transfected (A) and an _htrp3_-transfected (B) CPAE cell clamped at 0 mV and perfused with Krebs solution. The internal solution was a high-K+ internal solution containing 0·1 mM EGTA. ATP (3 μM) was added to the bath as indicated by the horizontal bars. C, comparison of [Ca2+]i measured at the resting, peak and plateau phases in non-transfected (□) and _htrp3_-transfected ( ) cells. [Ca2+]i was measured before and after application of 3 μM ATP. Plateau [Ca2+]i was measured 3 min after the end of ATP application. *P < 0·01, significantly different from non-transfected cells.
) cells. [Ca2+]i was measured before and after application of 3 μM ATP. Plateau [Ca2+]i was measured 3 min after the end of ATP application. *P < 0·01, significantly different from non-transfected cells.
Figure 4. Non-selective cation currents in _htrp3_-transfected cells
A and B, membrane currents at -50 mV in control (A) and _htrp3_-transfected (B) CPAE cells before and during stimulation with 3 μM ATP. The internal Cs+-containing pipette solution was buffered at 100 nM Ca2+. Bath solution was a 1·5 mM Ca2+-containing Krebs solution, which was replaced by a Ca2+-free Krebs solution (middle bar) before stimulating the cells with ATP, as indicated by the upper bar. The lower bar indicates the replacement of extracellular Na+ with NMDG+. Note the presence in both cell types of an inward current component under resting conditions, which is enhanced in Ca2+-free solution and eliminated by replacing extracellular Na+ with NMDG+. ATP did not induce any additional current in the control cell, but induced a huge current in the transfected cell, which again was abolished in Na+-free solution. C, I-V relationships of the _htrp3_-transfected cells obtained from voltage ramps applied at the time points marked in B.
Figure 5. Comparison of hTRP3 currents under various experimental conditions
Comparison of the densities of Na+ influx currents in _htrp3_-transfected CPAE cells after the addition of 2 or 10 μM Ins_P_3, 100 nM bradykinin or 10 μM ATP. The currents were measured at -50 mV in cells dialysed with the internal solutions, which were buffered with 5 mM EGTA at either 100 nM [Ca2+]i or 1 μM [Ca2+]i, not buffered at all (0·1 mM EGTA) or buffered with 12 mM BAPTA in the patch pipette. Numbers in parentheses, number of cells.
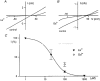
Figure 6. Block of _I_hTRP3 by the trivalent cations La3+ and Gd3+
A and B, comparison of the I-V curves of the current activated by 3 μM ATP before and after application of 100 μM La3+ or 100 μM Gd3+. C, concentration-response curve for the La3+ block. The ordinate represents the percentage of the control current at -50 mV that is not affected by La3+ or Gd3. The continuous line represents the best fit of the La3+ data to eqn (5) with an IC50 of 24·4 μM and _n_H= 1·42.
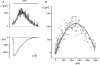
Figure 7. Relationship between current fluctuations and current amplitude
A, time course of current variance (upper trace) and current amplitude (lower trace) at a holding potential of -50 mV during application of 3 μM ATP. [Ca2+]i was buffered at 100 nM. B, current variance (σ2) as a function of current amplitude from the data in A. Parameters from the fit of the data to eqn (6) (continuous line) were _i_= 1·8 pA, _N_= 1420.
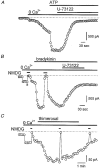
Figure 8. Effect of Ca2+-ATPase inhibitors on the membrane current of _htrp3_-transfected cells
A, effects of 20 μM BHQ (A) on a cell clamped at -50 mV using a pipette solution with Ca2+ buffered at 100 nM in a Na+-containing, Ca2+-free bath solution. B, same protocol but for 2 μM thapsigargin (TG). Note that 1 μM ATP could activate _I_hTRP3 in both cells whereas both Ca2+-ATPase inhibitors had no effect. The lower trace in B shows the intracellular Ca2+ concentration during this experiment.
Figure 9. Effects of U-73122, a PLC inhibitor, and thimerosal, a sensitizer of the Ins_P_3 receptor, on agonist-activated current in transfected cells
A and B, effects of 2 μM U-73122 on _I_hTRP3 activated by either 100 nM bradykinin or 3 μM ATP. The current was recorded at -50 mV; [Ca2+]i was buffered at 100 nM [Ca2+]i. The external bath solution contained Na+ as the major cation. C, effects of 5 μM thimerosal applied to the cell in a Ca2+-free solution. The membrane current was recorded at -50 mV with an internal high-Cs+ solution containing 0·1 mM EGTA and an external Na+ solution. Na+ was briefly replaced by NMDG+ to demonstrate the cationic nature of the current.
Similar articles
- Ca(2+)-dependent non-selective cation and potassium channels activated by bradykinin in pig coronary artery endothelial cells.
Baron A, Frieden M, Chabaud F, Bény JL. Baron A, et al. J Physiol. 1996 Jun 15;493 ( Pt 3)(Pt 3):691-706. doi: 10.1113/jphysiol.1996.sp021415. J Physiol. 1996. PMID: 8799892 Free PMC article. - Human Trp3 forms both inositol trisphosphate receptor-dependent and receptor-independent store-operated cation channels in DT40 avian B lymphocytes.
Vazquez G, Lievremont JP, St J Bird G, Putney JW Jr. Vazquez G, et al. Proc Natl Acad Sci U S A. 2001 Sep 25;98(20):11777-82. doi: 10.1073/pnas.201238198. Epub 2001 Sep 11. Proc Natl Acad Sci U S A. 2001. PMID: 11553786 Free PMC article. - Functional interaction between InsP3 receptors and store-operated Htrp3 channels.
Kiselyov K, Xu X, Mozhayeva G, Kuo T, Pessah I, Mignery G, Zhu X, Birnbaumer L, Muallem S. Kiselyov K, et al. Nature. 1998 Dec 3;396(6710):478-82. doi: 10.1038/24890. Nature. 1998. PMID: 9853757 - Nonselective cation channels in endothelial cells derived from human umbilical vein.
Kamouchi M, Mamin A, Droogmans G, Nilius B. Kamouchi M, et al. J Membr Biol. 1999 May 1;169(1):29-38. doi: 10.1007/pl00005898. J Membr Biol. 1999. PMID: 10227849 - Adenosine 5'-triphosphate: an intracellular metabolic messenger.
Szewczyk A, Pikuła S. Szewczyk A, et al. Biochim Biophys Acta. 1998 Jul 20;1365(3):333-53. doi: 10.1016/s0005-2728(98)00094-2. Biochim Biophys Acta. 1998. PMID: 9711292 Review. No abstract available.
Cited by
- Multiple roles of calmodulin and other Ca(2+)-binding proteins in the functional regulation of TRP channels.
Zhu MX. Zhu MX. Pflugers Arch. 2005 Oct;451(1):105-15. doi: 10.1007/s00424-005-1427-1. Epub 2005 May 28. Pflugers Arch. 2005. PMID: 15924238 Review. - Endothelial control of vasodilation: integration of myoendothelial microdomain signalling and modulation by epoxyeicosatrienoic acids.
Ellinsworth DC, Earley S, Murphy TV, Sandow SL. Ellinsworth DC, et al. Pflugers Arch. 2014 Mar;466(3):389-405. doi: 10.1007/s00424-013-1303-3. Epub 2013 Jun 8. Pflugers Arch. 2014. PMID: 23748495 Free PMC article. Review. - A diacylglycerol-activated Ca2+ channel in PC12 cells (an adrenal chromaffin cell line) correlates with expression of the TRP-6 (transient receptor potential) protein.
Tesfai Y, Brereton HM, Barritt GJ. Tesfai Y, et al. Biochem J. 2001 Sep 15;358(Pt 3):717-26. doi: 10.1042/0264-6021:3580717. Biochem J. 2001. PMID: 11535132 Free PMC article. - TRP channels in hypertension.
Firth AL, Remillard CV, Yuan JX. Firth AL, et al. Biochim Biophys Acta. 2007 Aug;1772(8):895-906. doi: 10.1016/j.bbadis.2007.02.009. Epub 2007 Mar 1. Biochim Biophys Acta. 2007. PMID: 17399958 Free PMC article. Review. - Histamine-induced Ca2+ oscillations in a human endothelial cell line depend on transmembrane ion flux, ryanodine receptors and endoplasmic reticulum Ca2+-ATPase.
Paltauf-Doburzynska J, Frieden M, Spitaler M, Graier WF. Paltauf-Doburzynska J, et al. J Physiol. 2000 May 1;524 Pt 3(Pt 3):701-13. doi: 10.1111/j.1469-7793.2000.00701.x. J Physiol. 2000. PMID: 10790152 Free PMC article.
References
- Bennett DL, Petersen CC, Cheek TR. Calcium signalling. Cracking ICRAC in the eye. Current Biology. 1995;5:1225–1228. - PubMed
- Birnbaumer L, Zhu X, Jiang M, Boulay G, Peyton M, Vannier B, Brown D, Platano D, Sadeghi H, Stefani E, Birnbaumer M. On the molecular basis and regulation of cellular capacitive calcium entry: Role for Trp proteins. Proceedings of the National Academy of Sciences of the USA. 1996;93:15195–15202. - PMC - PubMed
- Boulay G, Zhu X, Peyton M, Jiang M, Hurst R, Stefani E, Birnbaumer L. Cloning and expression of a novel mammalian homolog of Drosophila transient receptor potential (trp) involved in calcium entry secondary to activation of receptors coupled by the Gq class of G protein. Journal of Biological Chemistry. 1997;272:29672–29680. - PubMed
- Carter TD, Ogden D. Kinetics of intracellular calcium release by inositol 1,4,5-trisphosphate and extracellular ATP in porcine cultured aortic endothelial cells. Proceedings of the Royal Society. 1992;B 250:235–241. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous