Rapid report: postsynaptic bursting is essential for 'Hebbian' induction of associative long-term potentiation at excitatory synapses in rat hippocampus - PubMed (original) (raw)
Rapid report: postsynaptic bursting is essential for 'Hebbian' induction of associative long-term potentiation at excitatory synapses in rat hippocampus
F G Pike et al. J Physiol. 1999.
Abstract
1. The biologically relevant rules of synaptic potentiation were investigated in hippocampal slices from adult rat by mimicking neuronal activity seen during learning behaviours. Synaptic efficacy was monitored in two separate afferent pathways among the Schaffer collaterals during intracellular recording of CA1 pyramidal neurones. The effects of pairing presynaptic single spikes or bursts with postsynaptic single spikes or bursts, repeated at 5 Hz ('theta' frequency), were compared. 2. The pairing of ten single evoked excitatory synaptic events with ten postsynaptic single action potentials at 5 Hz, repeated twelve times, failed to induce synaptic enhancement (EPSP amplitude 95% of baseline amplitude 20 min after pairing; n = 5). In contrast, pairing the same number of action potentials, but clustered in bursts, induced robust synaptic potentiation (EPSP amplitude 163%; P < 0.01, Student's t test; n = 5). This potentiation was input specific, long lasting ( > 1 h; n = 3) and its induction was blocked by an antagonist at NMDA receptors (20-50 microM D(-)-2-amino-5-phosphonopentanoic acid; EPSP amplitude 109%; n = 6). 3. Presynaptic bursting paired with postsynaptic single action potentials did not induce input specific synaptic change (113 % in the test input vs. 111 % in the control; n = 8). In contrast, postsynaptic bursting when paired with presynaptic single action potentials was sufficient to induce synaptic potentiation when the presynaptic activity preceded the postsynaptic activity by 10 ms (150 vs. 84 % in the control input; P < 0.01; n = 10). 4. These results indicate that, under our conditions, postsynaptic bursting activity is necessary for associative synaptic potentiation at CA1 excitatory synapses in adult hippocampus. The existence of a distinct postsynaptic signal for induction of synaptic change calls for refinement of the common interpretation of Hebb's rule, and is likely to have important implications for our understanding of cortical network operation.
Figures
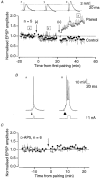
Figure 1. Necessity of burst firing for induction of synaptic potentiation
A, normalized EPSP amplitude monitored over time (mean of five experiments). No increase in synaptic efficacy was seen following pairing of single presynaptic stimuli with single postsynaptic action potentials (a), whereas robust potentiation was induced by pairing triple presynaptic stimuli with bursts of postsynaptic action potentials (b). •, control; ▵, paired input. Error bars indicate
s.e.m.
The total number of stimuli and action potentials were the same during the two pairings. Inset, analog test EPSPs from a typical experiment at the indicated time points (x, y and z). B, pairing protocols. a, single presynaptic stimuli (arrowhead) were paired with a single postsynaptic action potential elicited by an intracellular current pulse (1 nA, 5 ms). A train of ten such pairings was made at 5 Hz, and this train was repeated 12 times, at 10 s intervals. b, triple presynaptic stimuli at 200 Hz (arrowheads) were followed by an intracellular current pulse (1 nA, 20 ms) eliciting three postsynaptic action potentials. A train of ten such pairings was made at 5 Hz, and this train was repeated four times, at 10 s intervals. C, NMDA receptor dependence of burst pairing-induced potentiation. A set of experiments using the burst pairing protocol described above, performed during bath application of D(-)-2-amino-5-phosphonopentanoate (D-AP5), revealed no potentiation (_n_= 6). •, control; ▵, paired input. Error bars indicate
s.e.m.
Figure 2. Postsynaptic bursting as the essential signal during pairing-induced synaptic potentiation
Four different pairing protocols were compared using the same number of pairings: A, pairing single presynaptic stimuli with single postsynaptic action potentials; B, triple presynaptic stimuli at 200 Hz with postsynaptic single action potentials; C, single presynaptic stimuli with postsynaptic bursts; and D, triple presynaptic stimuli at 200 Hz with postsynaptic bursts. Each pairing was repeated 10 times at 5 Hz, and this train was repeated four times at 10 s intervals. Only protocols incorporating postsynaptic bursting (C and D) produced input-specific potentiation. Symbols and axes as Fig. 1_A_. Error bars indicate
s.e.m.
Similar articles
- Long-term change in synaptic transmission in CA3 circuits followed by spontaneous rhythmic activity in rat hippocampal slices.
Nakashima K, Hayashi H, Shimizu O, Ishizuka S. Nakashima K, et al. Neurosci Res. 2001 Aug;40(4):325-36. doi: 10.1016/s0168-0102(01)00244-9. Neurosci Res. 2001. PMID: 11463478 - Endogenous opioids regulate long-term potentiation of synaptic inhibition in the dentate gyrus of rat hippocampus.
Xie CW, Lewis DV. Xie CW, et al. J Neurosci. 1995 May;15(5 Pt 2):3788-95. doi: 10.1523/JNEUROSCI.15-05-03788.1995. J Neurosci. 1995. PMID: 7751946 Free PMC article. - C-type natriuretic peptide modulates bidirectional plasticity in hippocampal area CA1 in vitro.
Decker JM, Wójtowicz AM, Bartsch JC, Liotta A, Braunewell KH, Heinemann U, Behrens CJ. Decker JM, et al. Neuroscience. 2010 Aug 11;169(1):8-22. doi: 10.1016/j.neuroscience.2010.04.064. Epub 2010 May 8. Neuroscience. 2010. PMID: 20438814 - The role of dendritic filtering in associative long-term synaptic plasticity.
Sourdet V, Debanne D. Sourdet V, et al. Learn Mem. 1999 Sep-Oct;6(5):422-47. doi: 10.1101/lm.6.5.422. Learn Mem. 1999. PMID: 10541464 Review. - CaM kinase II in long-term potentiation.
Fukunaga K, Muller D, Miyamoto E. Fukunaga K, et al. Neurochem Int. 1996 Apr;28(4):343-58. doi: 10.1016/0197-0186(95)00097-6. Neurochem Int. 1996. PMID: 8740440 Review.
Cited by
- Dopamine regulates intrinsic excitability thereby gating successful induction of spike timing-dependent plasticity in CA1 of the hippocampus.
Edelmann E, Lessmann V. Edelmann E, et al. Front Neurosci. 2013 Mar 6;7:25. doi: 10.3389/fnins.2013.00025. eCollection 2013. Front Neurosci. 2013. PMID: 23508132 Free PMC article. - The influence of stress and gonadal hormones on neuronal structure and function.
Farrell MR, Gruene TM, Shansky RM. Farrell MR, et al. Horm Behav. 2015 Nov;76:118-24. doi: 10.1016/j.yhbeh.2015.03.003. Epub 2015 Mar 25. Horm Behav. 2015. PMID: 25819727 Free PMC article. Review. - Age-dependent changes in intrinsic neuronal excitability in subiculum after status epilepticus.
Chung S, Spruston N, Koh S. Chung S, et al. PLoS One. 2015 Mar 16;10(3):e0119411. doi: 10.1371/journal.pone.0119411. eCollection 2015. PLoS One. 2015. PMID: 25775210 Free PMC article. - Plasticity compartments in basal dendrites of neocortical pyramidal neurons.
Gordon U, Polsky A, Schiller J. Gordon U, et al. J Neurosci. 2006 Dec 6;26(49):12717-26. doi: 10.1523/JNEUROSCI.3502-06.2006. J Neurosci. 2006. PMID: 17151275 Free PMC article. - Long-term population spike-timing-dependent plasticity promotes synaptic tagging but not cross-tagging in rat hippocampal area CA1.
Pang KKL, Sharma M, Krishna-K K, Behnisch T, Sajikumar S. Pang KKL, et al. Proc Natl Acad Sci U S A. 2019 Mar 19;116(12):5737-5746. doi: 10.1073/pnas.1817643116. Epub 2019 Feb 28. Proc Natl Acad Sci U S A. 2019. PMID: 30819889 Free PMC article.
References
- Bland BH. The physiology and pharmacology of hippocampal formation theta rhythms. Progress in Neurobiology. 1986;26:1–54. - PubMed
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous