Dimerization of the Agrobacterium tumefaciens VirB4 ATPase and the effect of ATP-binding cassette mutations on the assembly and function of the T-DNA transporter - PubMed (original) (raw)
Dimerization of the Agrobacterium tumefaciens VirB4 ATPase and the effect of ATP-binding cassette mutations on the assembly and function of the T-DNA transporter
T A Dang et al. Mol Microbiol. 1999 Jun.
Abstract
The Agrobacterium tumefaciens VirB4 ATPase functions with other VirB proteins to export T-DNA to susceptible plant cells and other DNA substrates to a variety of prokaryotic and eukaryotic cells. Previous studies have demonstrated that VirB4 mutants with defects in the Walker A nucleotide-binding motif are non-functional and exert a dominant negative phenotype when synthesized in wild-type cells. This study characterized the oligomeric structure of VirB4 and examined the effects of Walker A sequence mutations on complex formation and transporter activity. VirB4 directed dimer formation when fused to the amino-terminal portion of cI repressor protein, as shown by immunity of Escherichia coli cells to lambda phage infection. VirB4 also dimerized in Agrobacterium tumefaciens, as demonstrated by the recovery of a detergent-resistant complex of native protein and a functional, histidine-tagged derivative by precipitation with anti-His6 antibodies and by Co2+ affinity chromatography. Walker A sequence mutants directed repressor dimerization in E. coli and interacted with His-VirB4 in A. tumefaciens, indicating that ATP binding is not required for self-association. A dimerization domain was localized to a proposed N-terminal membrane-spanning region of VirB4, as shown by the dominance of an allele coding for the N-terminal 312 residues and phage immunity of host cells expressing cI repressor fusions to alleles for the first 237 or 312 residues. A recent study reported that the synthesis of a subset of VirB proteins, including VirB4, in agrobacterial recipients has a pronounced stimulatory effect on the virB-dependent conjugal transfer of plasmid RSF1010 by agrobacterial donors. VirB4'312 suppressed the stimulatory effect of VirB proteins for DNA uptake when synthesized in recipient cells. In striking contrast, Walker A sequence mutants contributed to the stimulatory effect of VirB proteins to the same extent as native VirB4. These findings indicate that the oligomeric structure of VirB4, but not its capacity to bind ATP, is important for the assembly of VirB proteins as a DNA uptake system. The results of these studies support a model in which VirB4 dimers or homomultimers contribute structural information for the assembly of a transenvelope channel competent for bidirectional DNA transfer, whereas an ATP-dependent activity is required for configuring this channel as a dedicated export machine.
Figures
Fig. 1
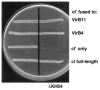
Immunity of E. coli AG1688 cells expressing cI chimeric genes. Strains were streaked across a line of λKH54 phage (vertical line) for the determination of phage-sensitivity phenotypes. Strains synthesizing cI′ fused to proteins indicated at the right carried the following expression plasmids: VirB11 (pTAD180), VirB4 (pTAD181), cI′ N-terminal 117 residues of cI (pSR58), cI full-length (pJH157).
Fig. 2
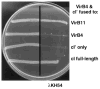
Immunity of E. coli AG1688 cells co-expressing wild-type virB4 and cI chimeric genes. VirB4 was synthesized from Plac carried on IncP plasmid pPC40. Proteins listed at the right were synthesized from the pBR322-based plasmids listed in Fig. 1.
Fig. 3
Immunoprecipitation (IP) of VirB4 complexes from detergent-solubilized A. tumefaciens extracts. Immunoprecipitates were analysed by SDS–PAGE and immunostaining with anti-VirB4 antibodies. Upper reactive species is the 89 kDa His-VirB4; lower species is the 87 kDa native VirB4 protein. Strains: wild-type A348 (lanes 1–3), PC1004(pTAD2141) (lanes 4–6), PC1000(pTAD2141) (lanes 7–9), PC1000(pTAD214) (lanes 10–12). Lanes 1, 4, 7 and 10 are cell extracts before IP; lanes 2, 5, 8 and 11 are supernatants after IP; lanes 3, 6, 9 and 12 are immunoprecipitates.
Fig. 4
Cobalt affinity chromatography of DM-solubilized A. tumefaciens extracts. Column fractions were analysed by SDS–PAGE and immunostaining with anti-VirB4 antibodies. Upper reactive species is the 89 kDa His-VirB4; lower species is the 87 kDa native VirB4 protein. A. Strains: wild-type A348 (lanes 1–3), PC1004(pTAD2141) (lanes 4–6), PC1000(pTAD2141) (lanes 7–9), PC1000(pTAD214) (lanes 10–12). B. Strains: PC1004(pBB15) (lanes 1–3), PC1004(pPC43) (lanes 4–6), PC1000(pPC43) (lanes 7–9). C. Strains: PC1004(pBB17) (lanes 1–3), PC1004(pPC44) (lanes 4–6), PC1000(pPC44) (lanes 7–9). Lanes 1, 4, 7 and 10 are cell extracts before chromatography; lanes 2, 5, 8 and 11 are flowthrough fractions; lanes 3, 6, 9 and 12 are fractions eluted with 100 mM imidazole.
Fig. 5
VirB4 derivatives and their phenotypes. A. Native VirB4 (789 residues) at the top with positions noted for proposed transmembrane regions 1 and 2, with dark stipples denoting proposed periplasmic loops and light stipples denoting flanking transmembrane segments. The Walker A nucleotide-binding sequence is denoted by a black box. The His tag is denoted with a box filled with diagonal lines. Synthesis of detectable protein was assessed by immunostaining. Functionality was assessed by the ability to restore virulence to strain PC1004. Dominance was assessed by the virulence of A348 merodiploids. B. Virulence assays showing the dominance of VirB4 truncation proteins. Strains synthesized native VirB4 (top left), His-VirB4 (top right) or both native VirB4 and the truncated proteins listed (two side-by-side inoculations per strain).
Fig. 6
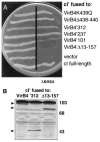
Immunity of E. coli AG1688 cells expressing _cI_′ fused to virB4 deletions. A. Strains synthesizing cI′ fused to proteins indicated at the right carried the following expression plasmids: VirB4K439Q (pPC45), VirB4Δ438–440 (pPC46), VirB4′312 (pTAD182), VirB4′237 (pTAD183), VirB4′101 (pTAD184), VirB4′Δ13–157 (pTAD185), vector (pSR58), cI full-length (pJH157). B. Immunoblot analysis of extracts from AG1688 strains synthesizing cI′ fused to the VirB4 derivatives listed. Blots developed with anti-VirB4 antisera show the presence of hybrid proteins (indicated by arrowheads) of the expected sizes. VirB4 antisera cross-reacted with a high-molecular-weight species (> 103 kDa) in the AG1688 cell extracts.
Similar articles
- Self-assembly of the Agrobacterium tumefaciens VirB11 traffic ATPase.
Rashkova S, Zhou XR, Chen J, Christie PJ. Rashkova S, et al. J Bacteriol. 2000 Aug;182(15):4137-45. doi: 10.1128/JB.182.15.4137-4145.2000. J Bacteriol. 2000. PMID: 10894719 Free PMC article. - Role of Agrobacterium VirB11 ATPase in T-pilus assembly and substrate selection.
Sagulenko E, Sagulenko V, Chen J, Christie PJ. Sagulenko E, et al. J Bacteriol. 2001 Oct;183(20):5813-25. doi: 10.1128/JB.183.20.5813-5825.2001. J Bacteriol. 2001. PMID: 11566978 Free PMC article. - Promiscuous DNA transfer system of Agrobacterium tumefaciens: role of the virB operon in sex pilus assembly and synthesis.
Kado CI. Kado CI. Mol Microbiol. 1994 Apr;12(1):17-22. doi: 10.1111/j.1365-2958.1994.tb00990.x. Mol Microbiol. 1994. PMID: 7914664 Review. - The Agrobacterium VirB/VirD4 T4SS: Mechanism and Architecture Defined Through In Vivo Mutagenesis and Chimeric Systems.
Li YG, Christie PJ. Li YG, et al. Curr Top Microbiol Immunol. 2018;418:233-260. doi: 10.1007/82_2018_94. Curr Top Microbiol Immunol. 2018. PMID: 29808338 Free PMC article. Review.
Cited by
- Membrane association and polar localization of the Legionella pneumophila T4SS DotO ATPase mediated by two nonredundant receptors.
Vijayrajratnam S, Milek S, Maggi S, Ashen K, Ferrell M, Hasanovic A, Holgerson A, Kannaiah S, Singh M, Ghosal D, Jensen GJ, Vogel JP. Vijayrajratnam S, et al. Proc Natl Acad Sci U S A. 2024 Oct 8;121(41):e2401897121. doi: 10.1073/pnas.2401897121. Epub 2024 Oct 1. Proc Natl Acad Sci U S A. 2024. PMID: 39352935 - Bacterial One- and Two-Hybrid Assays to Monitor Transmembrane Helix Interactions.
Zoued A, Duneau JP, Cascales E. Zoued A, et al. Methods Mol Biol. 2024;2715:259-271. doi: 10.1007/978-1-0716-3445-5_17. Methods Mol Biol. 2024. PMID: 37930534 - Characterization of ConE, the VirB4 Homolog of the Integrative and Conjugative Element ICE_Bs1_ of Bacillus subtilis.
Murthy AC, Aleksanyan N, Morton GM, Toyoda HC, Kalashyan M, Chen S, Ragucci AE, Broulidakis MP, Swerdlow KJ, Bui MNN, Muccioli M, Berkmen MB. Murthy AC, et al. J Bacteriol. 2023 Jun 27;205(6):e0003323. doi: 10.1128/jb.00033-23. Epub 2023 May 23. J Bacteriol. 2023. PMID: 37219457 Free PMC article. - A unique cytoplasmic ATPase complex defines the Legionella pneumophila type IV secretion channel.
Chetrit D, Hu B, Christie PJ, Roy CR, Liu J. Chetrit D, et al. Nat Microbiol. 2018 Jun;3(6):678-686. doi: 10.1038/s41564-018-0165-z. Epub 2018 May 21. Nat Microbiol. 2018. PMID: 29784975 Free PMC article. - Dimerization of VirD2 binding protein is essential for Agrobacterium induced tumor formation in plants.
Padavannil A, Jobichen C, Qinghua Y, Seetharaman J, Velazquez-Campoy A, Yang L, Pan SQ, Sivaraman J. Padavannil A, et al. PLoS Pathog. 2014 Mar 13;10(3):e1003948. doi: 10.1371/journal.ppat.1003948. eCollection 2014 Mar. PLoS Pathog. 2014. PMID: 24626239 Free PMC article.
References
- Akopyants NS, Clifton SW, Kersulyte D, Crabtree JE, Youree BE, Reece CA, et al. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol Microbiol. 1998;28:37–53. - PubMed
- Anderson SGE, Zomorodipour A, Andersson JO, Sicheritz-Ponten T, Alsmark UCM, Podowski RM, et al. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature. 1998;396:133–140. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources