In vivo pathway of thermal hyperalgesia by intrathecal administration of alpha,beta-methylene ATP in mouse spinal cord: involvement of the glutamate-NMDA receptor system - PubMed (original) (raw)
In vivo pathway of thermal hyperalgesia by intrathecal administration of alpha,beta-methylene ATP in mouse spinal cord: involvement of the glutamate-NMDA receptor system
M Tsuda et al. Br J Pharmacol. 1999 May.
Abstract
1. The aim of the present study is to characterize the role of the P2X receptor in spinal nociceptive processing in vivo. We investigated the mechanisms of the P2X receptor agonist alpha,beta-methylene ATP (alpha,betameATP)-induced modulation of acute nociceptive signalling in mouse spinal cord. 2. Intrathecal administration of alpha,betameATP produced a significant and dose-dependent thermal hyperalgesic response. This response was completely blocked by intrathecal pretreatment with the non-selective P2 receptor antagonist, pyridoxal-phosphate-6-azophenyl-2',4'-disulphonate (PPADS) and the selective P2X1, P2X3 and P2X2-3 receptor antagonist, 2',3'-O-(2,4,6-trinitrophenyl)adenosine 5'-triphosphate (TNP-ATP). Pretreatment with alpha,betameATP 15, 30 and 60 min prior to administration of a second dose of alpha,betameATP diminished the alpha,betameATP-induced thermal hyperalgesia. 3. A potent agonist for the P2X1 receptor, beta,gamma-methylene-L-ATP, did not show the hyperalgesic response, indicating that the P2X1 receptor is not involved in the spinal nociceptive pathway. 4. In fura-2 experiments using mouse dorsal root ganglion (DRG) neurons, alpha,betameATP (100 microM) increased intracellular Ca2+ ([Ca2+]i). This was not produced by a second application of alpha,betameATP. The same DRG neurons also showed a marked [Ca2+]i increase in response to capsaicin (3 microM). 5. Intrathecal pretreatment with the Ca2+-dependent exocytosis inhibitor, botulinum neurotoxin B, abolished the thermal hyperalgesia by alpha,betameATP. Furthermore, thermal hyperalgesia was significantly inhibited by the N-methyl-D-aspartate (NMDA) receptor antagonists, 2-amino-5-phosphonopentanoate (APV), dizocilpine and ifenprodil. 6. These findings suggest that alpha,betameATP-induced thermal hyperalgesia may be mediated by the spinal P2X3 receptor subtype that causes unresponsiveness by repetitive agonist applications, and that alpha,betameATP (perhaps through P2X3 receptors) may evoke spinal glutamate release which, in turn, leads to the generation of thermal hyperalgesia via activation of NMDA receptors.
Figures
Figure 1
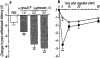
An intrathecal administration of α,βmeATP caused the thermal hyperalgesic response in mice. (a) Dose-response and (b) time-course of the thermal hyperalgesic response by intrathecal α,βmeATP injection. (a) Paw withdrawal response was measured 5 min after intrathecal injection of α,βmeATP (1.0–5.0 μg per mouse; dotted columns) or PBS (5 μl; open column). (b) Paw withdrawal response was measured 0, 5, 15, 30 and 60 min after intrathecal injection of α,βmeATP (5.0 μg per mouse; closed circle) or PBS (5 μl; open circle). Ordinate: change in paw withdrawal latency (s; latency of paw withdrawal response after α,βmeATP or PBS injection minus latency before that). Each point and column represent the mean±s.e.mean of 10 mice. **P<0.01 vs PBS-treated group.
Figure 2
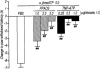
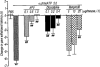
Effect of the antagonists for the non-selective P2 receptor PPADS and the selective P2X1, P2X3 and P2X2+3 receptor TNP-ATP on the thermal hyperalgesic response by intrathecal injection of α,βmeATP in mice. Mice were injected intrathecal with PPADS (1.0–5.0 μg per mouse; hatched columns) or TNP-ATP (0.25–1.0 μg per mouse; closed columns) 10 min prior to intrathecal injection of α,βmeATP (5.0 μg per mouse; open column). Paw withdrawal response was measured 5 min after injection with α,βmeATP. Ordinate: change in paw withdrawal latency(s). Each column represents the mean±s.e.mean of 7–11 mice. ##P<0.01 vs α,βmeATP (5.0 μg per mouse)-injected control group.
Figure 3
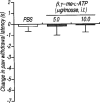
Effect of the intrathecal injection of β,γme-
L
-ATP on the paw withdrawal latency in mice. The paw withdrawal response was measured 5 min after intrathecal injection of β,γ me-
L
-ATP (5.0 and 10.0 μg per mouse; dotted columns) or PBS (5 μl; open column). Ordinate: change in paw withdrawal latency(s). Each column represents the mean±s.e.mean of 5–7 mice.
Figure 4
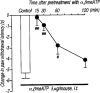
Effect of the intrathecal pretreatment with α,βmeATP on the thermal hyperalgesic response by intrathecal injection of α,βmeATP in mice. Mice were injected intrathecal with α,βmeATP (5.0 μg per mouse) 15, 30, 60 and 120 min prior to intrathecal injection of α,βmeATP (5.0 μg per mouse) (closed circles). Paw withdrawal response was measured 5 min after injection with α,βmeATP. Ordinate: change in paw withdrawal latency(s). Each column represents the mean±s.e.mean of 8–12 mice. #P<0.05, ##P<0.01 vs α,βmeATP (5.0 μg per mouse)-injected control group.
Figure 5
Effects of α,βmeATP on [Ca2+] in acutely dissociated DRG neuron from adult mouse. Horizontal solid bars show the applications of α,βmeATP (100 μ
M
) and capsaicin (3 μ
M
) for 15 s. A second application of α,βmeATP (100 μ
M
) was applied 5 min after the first application.
Figure 6
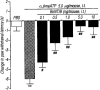
Effects of pretreatment with botulinum neurotoxin type B (BoNT/B) on the thermal hyperalgesic response by intrathecal injection of α,βmeATP in mice. Mice were injected intrathecal with BoNT/B (0.1–10.0 ng per mouse; closed columns) 12 h prior to intrathecal injection of α,βmeATP (5.0 μg per mouse). Paw withdrawal response was measured 5 min after the injection of α,βmeATP or PBS. Ordinate: change in paw withdrawal latency(s). Each column represents the mean±s.e.mean of 7–12 mice. **P<0.01 vs PBS-injected group; #P<0.05, ##P<0.01 vs α,βmeATP (5.0 μg per mouse)-injected control group.
Figure 7
Effects of the pretreatment with NMDA receptor antagonists on the thermal hyperalgesic response by intrathecal injection of α,βmeATP in mice. Mice were injected intrathecal with APV (0.1–1.0 μg per mouse: hatched columns), dizocilpine (0.1–0.4 μg per mouse; closed columns) and ifenprodil (5–20 μg per mouse; cross hatched columns) 10 min prior to the intrathecal injection of α,βmeATP (5.0 μg per mouse). Paw withdrawal response was measured 5 min after the injection of α,βmeATP or PBS. Ordinate: change in paw withdrawal latency(s). Each column represents the mean±s.e.mean of 6–18 mice. **P<0.01 vs PBS-injected group; ##P<0.01 vs α,βmeATP (5.0 μg per mouse)-injected control group.
Similar articles
- Evidence for the involvement of spinal endogenous ATP and P2X receptors in nociceptive responses caused by formalin and capsaicin in mice.
Tsuda M, Ueno S, Inoue K. Tsuda M, et al. Br J Pharmacol. 1999 Dec;128(7):1497-504. doi: 10.1038/sj.bjp.0702960. Br J Pharmacol. 1999. PMID: 10602329 Free PMC article. - Mechanical allodynia caused by intraplantar injection of P2X receptor agonist in rats: involvement of heteromeric P2X2/3 receptor signaling in capsaicin-insensitive primary afferent neurons.
Tsuda M, Koizumi S, Kita A, Shigemoto Y, Ueno S, Inoue K. Tsuda M, et al. J Neurosci. 2000 Aug 1;20(15):RC90. doi: 10.1523/JNEUROSCI.20-15-j0007.2000. J Neurosci. 2000. PMID: 10899177 Free PMC article. - TNP-ATP-resistant P2X ionic current on the central terminals and somata of rat primary sensory neurons.
Tsuzuki K, Ase A, Séguéla P, Nakatsuka T, Wang CY, She JX, Gu JG. Tsuzuki K, et al. J Neurophysiol. 2003 Jun;89(6):3235-42. doi: 10.1152/jn.01171.2002. J Neurophysiol. 2003. PMID: 12783957 - [ATP receptors in pain].
Tsuda M, Koizumi S, Inoue K. Tsuda M, et al. Nihon Yakurigaku Zasshi. 2000 Dec;116(6):343-50. doi: 10.1254/fpj.116.343. Nihon Yakurigaku Zasshi. 2000. PMID: 11188502 Review. Japanese. - Nitric oxide (NO) and nociceptive processing in the spinal cord.
Meller ST, Gebhart GF. Meller ST, et al. Pain. 1993 Feb;52(2):127-136. doi: 10.1016/0304-3959(93)90124-8. Pain. 1993. PMID: 8455960 Review.
Cited by
- Behavioral phenotypes of mice lacking purinergic P2X4 receptors in acute and chronic pain assays.
Tsuda M, Kuboyama K, Inoue T, Nagata K, Tozaki-Saitoh H, Inoue K. Tsuda M, et al. Mol Pain. 2009 Jun 11;5:28. doi: 10.1186/1744-8069-5-28. Mol Pain. 2009. PMID: 19515262 Free PMC article. - Mechanism of stretch-activated excitatory and inhibitory responses in the lower esophageal sphincter.
Jiang Y, Bhargava V, Mittal RK. Jiang Y, et al. Am J Physiol Gastrointest Liver Physiol. 2009 Aug;297(2):G397-405. doi: 10.1152/ajpgi.00108.2009. Epub 2009 Jun 11. Am J Physiol Gastrointest Liver Physiol. 2009. PMID: 19520741 Free PMC article. - Eugenol Inhibits ATP-induced P2X Currents in Trigeminal Ganglion Neurons.
Li HY, Lee BK, Kim JS, Jung SJ, Oh SB. Li HY, et al. Korean J Physiol Pharmacol. 2008 Dec;12(6):315-21. doi: 10.4196/kjpp.2008.12.6.315. Epub 2008 Dec 31. Korean J Physiol Pharmacol. 2008. PMID: 19967073 Free PMC article. - The effect of selective serotonin reuptake inhibitor (SSRI) on pain-related behavior in a rat model of neuropathic pain.
Saito H, Wakai J, Sekiguchi M, Kikuchi S, Konno S. Saito H, et al. Eur Spine J. 2014 Nov;23(11):2401-9. doi: 10.1007/s00586-014-3392-x. Epub 2014 Jun 5. Eur Spine J. 2014. PMID: 24898312 - Pharmacology of P2X channels.
Gever JR, Cockayne DA, Dillon MP, Burnstock G, Ford AP. Gever JR, et al. Pflugers Arch. 2006 Aug;452(5):513-37. doi: 10.1007/s00424-006-0070-9. Epub 2006 Apr 29. Pflugers Arch. 2006. PMID: 16649055 Review.
References
- AANONSEN L.M., WILCOX G.L. Nociceptive action of excitatory amino acids in the mouse: effects of spinally administered opioids, phencylidine and sigma agonists. J. Pharmacol. Exp. Ther. 1987;243:9–19. - PubMed
- ALMEIDA M.T., RAMALHO-SANTOS J., OLIVEIRA C.R., PEDROSO DE LIMA M.C. Evidence that synaptobrevin is involved in fusion between synaptic vesicle and synaptic plasma membrane vesicle. Biochem. Biophys. Res. Comm. 1997;236:184–188. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous