Three v-SNAREs and two t-SNAREs, present in a pentameric cis-SNARE complex on isolated vacuoles, are essential for homotypic fusion - PubMed (original) (raw)
Three v-SNAREs and two t-SNAREs, present in a pentameric cis-SNARE complex on isolated vacuoles, are essential for homotypic fusion
C Ungermann et al. J Cell Biol. 1999.
Abstract
Vacuole SNAREs, including the t-SNAREs Vam3p and Vam7p and the v-SNARE Nyv1p, are found in a multisubunit "cis" complex on isolated organelles. We now identify the v-SNAREs Vti1p and Ykt6p by mass spectrometry as additional components of the immunoisolated vacuolar SNARE complex. Immunodepletion of detergent extracts with anti-Vti1p removes all the Ykt6p that is in a complex with Vam3p, immunodepletion with anti-Ykt6p removes all the Vti1p that is complexed with Vam3p, and immunodepletion with anti-Nyv1p removes all the Ykt6p in complex with other SNAREs, demonstrating that they are all together in the same cis multi-SNARE complex. After priming, which disassembles the cis-SNARE complex, antibodies to any of the five SNARE proteins still inhibit the fusion assay until the docking stage is completed, suggesting that each SNARE plays a role in docking. Furthermore, vti1 temperature-sensitive alleles cause a synthetic fusion-defective phenotype in our reaction. Our data show that vacuole-vacuole fusion requires a cis-SNARE complex of five SNAREs, the t-SNAREs Vam3p and Vam7p and the v-SNAREs Nyv1p, Vti1p, and Ykt6p.
Figures
Figure 1
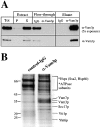
Identification of Vti1p and Ykt6p in a complex with Vam3p. Vacuoles (26 μg) were purified and detergent solubilized in 10 ml of buffer A as described in Materials and Methods. Aliquots (50 μl) were removed for analysis of the lysate before (Tot) and after centrifugation (S). The insoluble pellet was resuspended in 10 ml of lysate buffer and 50 μl was removed (P). Proteins were TCA-precipitated, washed with ice-cold acetone, and resuspended in SDS-sample buffer. The detergent extract was preadsorbed with nonimmune IgGs as described in Materials and Methods, then halved and incubated with a control IgG resin and with protein A–immobilized affinity-purified anti-Vam3p antibodies. An aliquot of the flow through (50 μl) from each column was saved and proteins were TCA-precipitated, acetone-washed, and resuspended in sample buffer as above. The columns were washed and specifically retained proteins were eluted as described in Materials and Methods, precipitated by TCA, washed with 1 ml ice-cold acetone, and dissolved in 50 μl of SDS-sample buffer. Aliquots of Tot, P, S, and the flow through together with 2 μl of the eluted samples were analyzed by SDS-PAGE, transferred to nitrocellulose, and immunoblotted for Vam3p (A). The remaining eluted proteins from both columns were also analyzed by SDS-PAGE and stained with Coomassie brilliant blue (B). Specific protein bands were excised and trypsin digested, and the resulting peptide mixtures were analyzed by MALDI mass spectrometry. Identified proteins are indicated. The peptide mass maps of Vti1p (C) (6 peptides, 30% amino acid sequence coverage) and Ykt6p (D) (8 tryptic peptides, 53% amino acid sequence coverage) are shown.
Figure 1
Identification of Vti1p and Ykt6p in a complex with Vam3p. Vacuoles (26 μg) were purified and detergent solubilized in 10 ml of buffer A as described in Materials and Methods. Aliquots (50 μl) were removed for analysis of the lysate before (Tot) and after centrifugation (S). The insoluble pellet was resuspended in 10 ml of lysate buffer and 50 μl was removed (P). Proteins were TCA-precipitated, washed with ice-cold acetone, and resuspended in SDS-sample buffer. The detergent extract was preadsorbed with nonimmune IgGs as described in Materials and Methods, then halved and incubated with a control IgG resin and with protein A–immobilized affinity-purified anti-Vam3p antibodies. An aliquot of the flow through (50 μl) from each column was saved and proteins were TCA-precipitated, acetone-washed, and resuspended in sample buffer as above. The columns were washed and specifically retained proteins were eluted as described in Materials and Methods, precipitated by TCA, washed with 1 ml ice-cold acetone, and dissolved in 50 μl of SDS-sample buffer. Aliquots of Tot, P, S, and the flow through together with 2 μl of the eluted samples were analyzed by SDS-PAGE, transferred to nitrocellulose, and immunoblotted for Vam3p (A). The remaining eluted proteins from both columns were also analyzed by SDS-PAGE and stained with Coomassie brilliant blue (B). Specific protein bands were excised and trypsin digested, and the resulting peptide mixtures were analyzed by MALDI mass spectrometry. Identified proteins are indicated. The peptide mass maps of Vti1p (C) (6 peptides, 30% amino acid sequence coverage) and Ykt6p (D) (8 tryptic peptides, 53% amino acid sequence coverage) are shown.
Figure 2
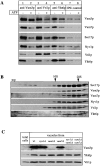
Vti1p and Ykt6p copurify with other members of the SNARE complex. (A) Coimmunoprecipitation analysis. Salt-washed vacuoles (100 μg) were resuspended in 750 μl of reaction buffer containing 1 mg/ml cytosol with or without ATP, 1.26 μg/ml his6-Sec18p, and an ATP-regenerating system (Haas, 1995). Reactions were incubated for 10 min at 27°C, vacuoles were reisolated (4 min, 8,000 g, 4°C), washed with 500 μl PS buffer, and detergent solubilized for 10 min at 0°C by adding 1 ml of lysis buffer (1% digitonin, 50 mM NaCl, 2 mM EDTA, 1 mM PMSF, 30 μM pefablock SC, 30 ng/ml leupeptin, 15 μM _o_-phenanthroline, 150 ng/ ml pepstatin). Insoluble material was removed by centrifugation (10 min, 20,000 g, 4°C) and protein complexes were analyzed by coimmunoprecipitation with protein A–immobilized antibodies to Vam3p, Vti1p (Fischer von Mollard et al., 1997), and Ykt6p as described (Ungermann et al., 1998). A portion (10%) of the total detergent extract was TCA precipitated. Proteins retained on the protein A–immunoglobulin beads were released by addition of 1 ml 0.1 M glycine/Cl, pH 2.6, precipitated by TCA, washed with ice-cold acetone and solubilized in SDS-sample buffer, analyzed by SDS-PAGE, transferred to nitrocellulose, and decorated with antibodies to Sec17p, Vam3p, Vam7p, Vti1p, Ykt6p, and Nyv1p. A greater percentage of Vti1p was typically dissociated from Ykt6p upon incubation with ATP than seen here (lanes 5 and 6; see, for example, lanes 3 and 4). (B) Vacuolar SNAREs comigrate on a glycerol gradient. Isolated wild-type vacuoles (300 μg) were solubilized for 10 min on ice as in A. The cleared detergent extract was loaded on a linear 10–34% glycerol gradient. The samples were centrifuged for 18 h at 4°C (40,000 rpm, Beckman SW41). Fractions (500 μl) were collected from top to bottom, proteins were precipitated with TCA, analyzed by SDS-PAGE, transferred to nitrocellulose, and decorated with antibodies to Vam3p, Vam7p, Sec17p, Ykt6p, Nyv1p, and Vti1p. The bottom of the gradient did not contain any detectable protein complexes and is not shown here. (C) Localization of Ykt6p and Vti1p to the vacuole. Proteins from total yeast extract and isolated wild-type and mutant vacuoles were resolved by SDS-PAGE and analyzed as in B. The Vam3p band in the total extract shows up only after long overexposure (data not shown), demonstrating the enrichment of Vam3p in the vacuolar fraction.
Figure 3
The cis-SNARE complex is pentameric for SNAREs. Salt-washed vacuoles (100 μg) were detergent solubilized by incubation for 10 min at 0°C in 1 ml of lysis buffer. Insoluble material was removed by centrifugation (10 min, 20,000 g, 4°C) and protein complexes were analyzed by coimmunoprecipitation with protein A–immobilized antibodies to Vam3p, Vti1p, Ykt6p, and Nyv1p as described (Ungermann et al., 1998a). A portion (10%) of the total detergent extract was TCA precipitated (lanes 6 and 8). Supernatants of the first immunoprecipitation were incubated for another 2 h at 4°C with protein A–immobilized IgGs to Ykt6p (lanes 3 and 7) or Vti1p (lane 5) and washed as described (Ungermann et al., 1998a). The immunoprecipitations shown in lanes 1–6 and lanes 7–9 were done in independent experiments. Proteins retained on the protein A–immunoglobulin beads in the first or second immunoprecipitation were analyzed as described in Fig. 2.
Figure 4
Vti1p and Ykt6p are part of the complex required for homotypic vacuole fusion. (A) Coincident acquisition of resistance to anti-Vam3p and anti-Vti1p during the fusion reaction. A 30-fold scale fusion reaction was started in the presence of ATP at 27°C. Aliquots (30 μl) were removed at indicated times and placed on ice or added to antibodies to Sec17p, Vam3p, or Vti1p and incubated at 27°C. Fusion reactions were incubated for a total of 90 min at 27°C before being assayed for alkaline phosphatase activity. (B) Vti1p action depends on vacuole docking. Two 10-fold reactions with vacuoles from either BJ3505 or from DKY6281 were incubated at 27°C in the presence of ATP. At indicated times, 15-μl portions were removed from each reaction and combined in the presence of buffer or antibodies to either Sec17p, Vam3p, or Vti1p and incubated for 90 min at 27°C. One aliquot was combined in the presence of buffer and placed on ice for the remaining reaction period. After 90 min, all reactions were analyzed for fusion. (C) Ts alleles in Vti1p cause a synthetic fusion phenotype. Cells were grown at 26°C overnight. Vacuoles were isolated from all strains and diluted in PS buffer to 0.3 mg/ ml. BJ and DKY vacuoles with wild-type, vti1-1, or vti1-2 alleles (10 μl) were combined and incubated at the indicated temperatures for 5 or 10 min and then chilled on ice. Cytosol, the ATP-regenerating system, and salts were added in a 10-μl volume and reactions were incubated for 90 min at 27°C and assayed for alkaline phosphatase. Wild-type fusion was set to 100%. (D) As in A, but antibodies to Ykt6p were added at the indicated times. (E) As in B, but antibodies to Yktp were added at the indicated times as noted.
Similar articles
- The Saccharomyces cerevisiae v-SNARE Vti1p is required for multiple membrane transport pathways to the vacuole.
Fischer von Mollard G, Stevens TH. Fischer von Mollard G, et al. Mol Biol Cell. 1999 Jun;10(6):1719-32. doi: 10.1091/mbc.10.6.1719. Mol Biol Cell. 1999. PMID: 10359592 Free PMC article. - A soluble SNARE drives rapid docking, bypassing ATP and Sec17/18p for vacuole fusion.
Thorngren N, Collins KM, Fratti RA, Wickner W, Merz AJ. Thorngren N, et al. EMBO J. 2004 Jul 21;23(14):2765-76. doi: 10.1038/sj.emboj.7600286. Epub 2004 Jul 8. EMBO J. 2004. PMID: 15241469 Free PMC article. - Characterization of a novel yeast SNARE protein implicated in Golgi retrograde traffic.
Lupashin VV, Pokrovskaya ID, McNew JA, Waters MG. Lupashin VV, et al. Mol Biol Cell. 1997 Dec;8(12):2659-76. doi: 10.1091/mbc.8.12.2659. Mol Biol Cell. 1997. PMID: 9398683 Free PMC article. - SNAREs and membrane fusion in the Golgi apparatus.
Nichols BJ, Pelham HR. Nichols BJ, et al. Biochim Biophys Acta. 1998 Aug 14;1404(1-2):9-31. doi: 10.1016/s0167-4889(98)00044-5. Biochim Biophys Acta. 1998. PMID: 9714710 Review. - A new catch in the SNARE.
Pratelli R, Sutter JU, Blatt MR. Pratelli R, et al. Trends Plant Sci. 2004 Apr;9(4):187-95. doi: 10.1016/j.tplants.2004.02.007. Trends Plant Sci. 2004. PMID: 15063869 Review.
Cited by
- The vacuolar DHHC-CRD protein Pfa3p is a protein acyltransferase for Vac8p.
Smotrys JE, Schoenfish MJ, Stutz MA, Linder ME. Smotrys JE, et al. J Cell Biol. 2005 Sep 26;170(7):1091-9. doi: 10.1083/jcb.200507048. J Cell Biol. 2005. PMID: 16186255 Free PMC article. - Phosphorylation of Ykt6 SNARE Domain Regulates Its Membrane Recruitment and Activity.
Karuna M P, Witte L, Linnemannstoens K, Choezom D, Danieli-Mackay A, Honemann-Capito M, Gross JC. Karuna M P, et al. Biomolecules. 2020 Nov 16;10(11):1560. doi: 10.3390/biom10111560. Biomolecules. 2020. PMID: 33207719 Free PMC article. - SNARE proteins are required for macroautophagy.
Nair U, Jotwani A, Geng J, Gammoh N, Richerson D, Yen WL, Griffith J, Nag S, Wang K, Moss T, Baba M, McNew JA, Jiang X, Reggiori F, Melia TJ, Klionsky DJ. Nair U, et al. Cell. 2011 Jul 22;146(2):290-302. doi: 10.1016/j.cell.2011.06.022. Cell. 2011. PMID: 21784249 Free PMC article. - ULK1-mediated phosphorylation regulates the conserved role of YKT6 in autophagy.
Sánchez-Martín P, Kriegenburg F, Alves L, Adam J, Elsaesser J, Babic R, Mancilla H, Licheva M, Tascher G, Münch C, Eimer S, Kraft C. Sánchez-Martín P, et al. J Cell Sci. 2023 Feb 1;136(3):jcs260546. doi: 10.1242/jcs.260546. Epub 2023 Feb 9. J Cell Sci. 2023. PMID: 36644903 Free PMC article. - Organelle acidification negatively regulates vacuole membrane fusion in vivo.
Desfougères Y, Vavassori S, Rompf M, Gerasimaite R, Mayer A. Desfougères Y, et al. Sci Rep. 2016 Jul 1;6:29045. doi: 10.1038/srep29045. Sci Rep. 2016. PMID: 27363625 Free PMC article.
References
- Banfield DK, Lewis MJ, Pelham HRB. A SNARE-like protein required for traffic through the Golgi complex. Nature. 1995;375:806–809. - PubMed
- Christoforidis S, McBride HM, Burgoyne RD, Zerial M. The Rab5 effector EEA1 is a core component of endosomal docking. Nature. 1999;397:621–625. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM23377/GM/NIGMS NIH HHS/United States
- R01 GM032448/GM/NIGMS NIH HHS/United States
- R37 GM032448/GM/NIGMS NIH HHS/United States
- GM32448/GM/NIGMS NIH HHS/United States
- R01 GM023377/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases