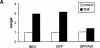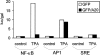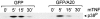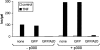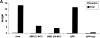The zinc finger protein A20 inhibits TNF-induced NF-kappaB-dependent gene expression by interfering with an RIP- or TRAF2-mediated transactivation signal and directly binds to a novel NF-kappaB-inhibiting protein ABIN - PubMed (original) (raw)
The zinc finger protein A20 inhibits TNF-induced NF-kappaB-dependent gene expression by interfering with an RIP- or TRAF2-mediated transactivation signal and directly binds to a novel NF-kappaB-inhibiting protein ABIN
K Heyninck et al. J Cell Biol. 1999.
Abstract
The zinc finger protein A20 is a tumor necrosis factor (TNF)- and interleukin 1 (IL-1)-inducible protein that negatively regulates nuclear factor-kappa B (NF-kappaB)-dependent gene expression. However, the molecular mechanism by which A20 exerts this effect is still unclear. We show that A20 does not inhibit TNF- induced nuclear translocation and DNA binding of NF-kappaB, although it completely prevents the TNF- induced activation of an NF-kappaB-dependent reporter gene, as well as TNF-induced IL-6 and granulocyte macrophage-colony stimulating factor gene expression. Moreover, NF-kappaB activation induced by overexpression of the TNF receptor-associated proteins TNF receptor-associated death domain protein (TRADD), receptor interacting protein (RIP), and TNF recep- tor-associated factor 2 (TRAF2) was also inhibited by expression of A20, whereas NF-kappaB activation induced by overexpression of NF-kappaB-inducing kinase (NIK) or the human T cell leukemia virus type 1 (HTLV-1) Tax was unaffected. These results demonstrate that A20 inhibits NF-kappaB-dependent gene expression by interfering with a novel TNF-induced and RIP- or TRAF2-mediated pathway that is different from the NIK-IkappaB kinase pathway and that is specifically involved in the transactivation of NF-kappaB. Via yeast two-hybrid screening, we found that A20 binds to a novel protein, ABIN, which mimics the NF-kappaB inhibiting effects of A20 upon overexpression, suggesting that the effect of A20 is mediated by its interaction with this NF-kappaB inhibiting protein, ABIN.
Figures
Figure 1
A20 inhibits NF-κB–dependent promoter activity without affecting NF-κB binding to DNA. (A) L929sA clones stably expressing the neomycin resistance gene alone or in combination with the plasmids encoding GFP or GFP/A20 as indicated were transiently transfected with a luciferase reporter gene under the control of three NF-κB recognition sequences [p(κB)3LUC]. Cells were either untreated (open bars) or treated with 1,000 IU/ ml mTNF (filled bars) for 6 h. Promoter activity is expressed as the luciferase (luc) activity relative to the β-galactosidase (gal) activity in order to correct for differences in transfection efficiency. Data from a representative experiment (total number of experiments, 2) are expressed as the mean value (n = 3) with SD < 10%. (B) The same transfectants were either untreated (−) or treated with 1,000 IU/ml mTNF (+) for 30 min. Nuclear extracts were analyzed for active NF-κB in an electrophoretic mobility shift assay as described in Materials and Methods.
Figure 1
A20 inhibits NF-κB–dependent promoter activity without affecting NF-κB binding to DNA. (A) L929sA clones stably expressing the neomycin resistance gene alone or in combination with the plasmids encoding GFP or GFP/A20 as indicated were transiently transfected with a luciferase reporter gene under the control of three NF-κB recognition sequences [p(κB)3LUC]. Cells were either untreated (open bars) or treated with 1,000 IU/ ml mTNF (filled bars) for 6 h. Promoter activity is expressed as the luciferase (luc) activity relative to the β-galactosidase (gal) activity in order to correct for differences in transfection efficiency. Data from a representative experiment (total number of experiments, 2) are expressed as the mean value (n = 3) with SD < 10%. (B) The same transfectants were either untreated (−) or treated with 1,000 IU/ml mTNF (+) for 30 min. Nuclear extracts were analyzed for active NF-κB in an electrophoretic mobility shift assay as described in Materials and Methods.
Figure 2
Subcellular localization of GFP and the fusion protein GFP/A20. Cells were seeded in coverglass chambers (Nunc) and GFP fluorescence was analyzed by excitation at 490 nm and emission between 510 and 525 nm using confocal microscopy (Zeiss). Transmission (A and B) and corresponding fluorescence images (C and D) are shown for the cell lines L929SA-GFP (A and C) and L929SA-GFP/A20 (B and D). No fluorescence was observed in L929SA-neo cells (data not shown).
Figure 3
Effect of A20 on NF-κB activation induced by TNF or overexpression of TRADD, RIP, TRAF2, and NIK in 293T cells. Cells were transfected with 1 μg plasmid DNA consisting of 300 ng empty plasmid or plasmids encoding TRADD, RIP, TRAF2, or NIK, 100 ng pUT651, 100 ng pNFconluc, 200 ng pCAGGS-GFP, or pCAGGS-GFP/A20, as well as 300 ng carrier plasmid DNA. Cells not expressing the NF-κB inducing proteins were left untreated or were treated for 6 h with 1,000 IU/ml hTNF. All cells were lysed 24 h after transfection and luciferase and β-galactosidase activities were measured. Promoter activity is expressed as the luciferase (luc) activity relative to the β-galactosidase (gal) activity. Data from a representative experiment (total number of experiments, 3) are expressed as the mean value (n = 3) with SD < 10%.
Figure 4
Effect of A20 on NF-κB activation by HTLV-1 Tax (A), IL-1 (B), or CD40 (C) in 293T cells. Cells were transfected with reporter gene constructs and GFP/A20 as described in the legend of Fig. 3. In A, NF-κB activation was induced by stimulation with 1,000 IU/ml hTNF for 6 h or by coexpression of an expression plasmid for HTLV-1 Tax (pIEX). In B, NF-κB activation was induced by stimulation with 7,000 IU/ml IL-1β for 6 h, whereas in C NF-κB activation was induced by coexpression of an expression plasmid for CD40 (pCDNA3-CD40). All cells were lysed 24 h after transfection and analyzed for luciferase expression as described in Materials and Methods. Data from a representative experiment (total number of experiments, 3) are expressed as the mean (n = 3) with SD < 10%.
Figure 5
Effect of A20 on TPA-induced activation of gene expression mediated by NF-κB, AP1, or SRE. 293T cells were transfected with 100 ng pUT651 and 100 ng NFconluc, pAP1-luc, or pSRE-luc, in combination with 100 ng pCAGGS-GFP or pCAGGS-GFP/A20. Cells were either left untreated or treated with 200 ng/ml TPA for 6 h. Cells were analyzed for luciferase expression as described in Materials and Methods. Data from a representative experiment (total number of experiments, 2) are expressed as the mean (n = 3) with SD < 10%.
Figure 6
A20 has no effect on TNF-induced p38 MAP kinase activation. L929sA-GFP and L929sA-GFP/A20 cells were left untreated or treated with 1,000 IU/ml mTNF during 5, 15, or 30 min and immediately lysed in SDS lysis buffer. Total lysates were separated on SDS-PAGE and activated p38 MAP kinase was detected by Western blotting using polyclonal antibodies recognizing specifically phosphorylated p38 MAP kinase. As a control for the amount of protein loaded, total expression of p38 MAP kinase was revealed with polyclonal anti-p38 MAP kinase antibodies and found to be identical in all samples (data not shown).
Figure 7
Effect of overexpression of p300 on TNF-induced NF-κB activation and its inhibition by A20. 293T cells were transfected with 100 ng pUT651 and 100 ng pNFconluc in combination with or without 100 ng pCMV-p300. 100 ng pCAGGS-GFP, pCAGGS-GFP/A20, or empty vector pCAGGS were cotransfected as indicated. 24 h after transfection, cells were either left untreated or treated with 1,000 IU/ml hTNF for 6 h. All cells were lysed and analyzed for luciferase expression as described in Materials and Methods. Data from a representative experiment (total number of experiments, 3) are expressed as the mean (n = 3) with SD < 10%.
Figure 8
Coimmunoprecipitation of A20 and ABIN after transient transfection of the encoding plasmids of E-tagged ABIN and GFP, GFP-A20, GFP-A20(369-775), GFP-A20(1-368), or an empty expression vector as a negative control in 293T cells. Immunoprecipitation (top) was performed with anti-GFP antibody and Western blot detection with anti–E-tag antibody. To control expression levels of ABIN, 10 μl of lysates was subjected to SDS-PAGE and Western blot detection with anti–E-tag antibody (bottom).
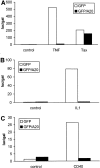
Figure 9
(A) Effect of two splice variants of ABIN [ABIN(1-647) and ABIN(54-647)] on the activation of NF-κB, measured by reporter gene activity. 293T cells were transiently transfected with 100 ng pUT651, 100 ng pNFconluc, and 200 ng expression plasmid and stimulated with TNF (1,000 IU/ml) during 6 h. As a control, plasmids encoding GFP and GFP-A20 were transfected. (B) Effect of transient transfection of suboptimal quantities of expression plasmids encoding A20 (5 ng) and ABIN (20 ng) on TNF-mediated NF-κB induction in 293T cells. (C) Effect of ABIN on NF-κB activation in 293T cells induced by overexpression of TRADD, RIP, TRAF2, NIK, or p65 after transfection of 300 ng of their encoding plasmids, together with 100 ng pUT651, 100 ng pNFconluc, and 500 ng pCAGGS-ABIN. (D) Effect of ABIN on NF-κB activation induced by overexpression of Tax in 293T cells. Cells were transfected with 1 μg plasmid DNA consisting of 300 ng empty plasmid or plasmid encoding Tax (pIEX), 100 ng pUT651, 100 ng pNFconluc, 200 ng pCAGGS or pCAGGS-ABIN, and 300 ng carrier plasmid DNA. All cells were lysed 30 h after transfection. In all experiments, cell extracts were analyzed for luciferase and β-galactosidase activity and the data are plotted as luc/gal, which is representative of NF-κB activity. Each value is the mean (n = 3) with SD < 10%.
Figure 9
(A) Effect of two splice variants of ABIN [ABIN(1-647) and ABIN(54-647)] on the activation of NF-κB, measured by reporter gene activity. 293T cells were transiently transfected with 100 ng pUT651, 100 ng pNFconluc, and 200 ng expression plasmid and stimulated with TNF (1,000 IU/ml) during 6 h. As a control, plasmids encoding GFP and GFP-A20 were transfected. (B) Effect of transient transfection of suboptimal quantities of expression plasmids encoding A20 (5 ng) and ABIN (20 ng) on TNF-mediated NF-κB induction in 293T cells. (C) Effect of ABIN on NF-κB activation in 293T cells induced by overexpression of TRADD, RIP, TRAF2, NIK, or p65 after transfection of 300 ng of their encoding plasmids, together with 100 ng pUT651, 100 ng pNFconluc, and 500 ng pCAGGS-ABIN. (D) Effect of ABIN on NF-κB activation induced by overexpression of Tax in 293T cells. Cells were transfected with 1 μg plasmid DNA consisting of 300 ng empty plasmid or plasmid encoding Tax (pIEX), 100 ng pUT651, 100 ng pNFconluc, 200 ng pCAGGS or pCAGGS-ABIN, and 300 ng carrier plasmid DNA. All cells were lysed 30 h after transfection. In all experiments, cell extracts were analyzed for luciferase and β-galactosidase activity and the data are plotted as luc/gal, which is representative of NF-κB activity. Each value is the mean (n = 3) with SD < 10%.
Figure 9
(A) Effect of two splice variants of ABIN [ABIN(1-647) and ABIN(54-647)] on the activation of NF-κB, measured by reporter gene activity. 293T cells were transiently transfected with 100 ng pUT651, 100 ng pNFconluc, and 200 ng expression plasmid and stimulated with TNF (1,000 IU/ml) during 6 h. As a control, plasmids encoding GFP and GFP-A20 were transfected. (B) Effect of transient transfection of suboptimal quantities of expression plasmids encoding A20 (5 ng) and ABIN (20 ng) on TNF-mediated NF-κB induction in 293T cells. (C) Effect of ABIN on NF-κB activation in 293T cells induced by overexpression of TRADD, RIP, TRAF2, NIK, or p65 after transfection of 300 ng of their encoding plasmids, together with 100 ng pUT651, 100 ng pNFconluc, and 500 ng pCAGGS-ABIN. (D) Effect of ABIN on NF-κB activation induced by overexpression of Tax in 293T cells. Cells were transfected with 1 μg plasmid DNA consisting of 300 ng empty plasmid or plasmid encoding Tax (pIEX), 100 ng pUT651, 100 ng pNFconluc, 200 ng pCAGGS or pCAGGS-ABIN, and 300 ng carrier plasmid DNA. All cells were lysed 30 h after transfection. In all experiments, cell extracts were analyzed for luciferase and β-galactosidase activity and the data are plotted as luc/gal, which is representative of NF-κB activity. Each value is the mean (n = 3) with SD < 10%.
Figure 9
(A) Effect of two splice variants of ABIN [ABIN(1-647) and ABIN(54-647)] on the activation of NF-κB, measured by reporter gene activity. 293T cells were transiently transfected with 100 ng pUT651, 100 ng pNFconluc, and 200 ng expression plasmid and stimulated with TNF (1,000 IU/ml) during 6 h. As a control, plasmids encoding GFP and GFP-A20 were transfected. (B) Effect of transient transfection of suboptimal quantities of expression plasmids encoding A20 (5 ng) and ABIN (20 ng) on TNF-mediated NF-κB induction in 293T cells. (C) Effect of ABIN on NF-κB activation in 293T cells induced by overexpression of TRADD, RIP, TRAF2, NIK, or p65 after transfection of 300 ng of their encoding plasmids, together with 100 ng pUT651, 100 ng pNFconluc, and 500 ng pCAGGS-ABIN. (D) Effect of ABIN on NF-κB activation induced by overexpression of Tax in 293T cells. Cells were transfected with 1 μg plasmid DNA consisting of 300 ng empty plasmid or plasmid encoding Tax (pIEX), 100 ng pUT651, 100 ng pNFconluc, 200 ng pCAGGS or pCAGGS-ABIN, and 300 ng carrier plasmid DNA. All cells were lysed 30 h after transfection. In all experiments, cell extracts were analyzed for luciferase and β-galactosidase activity and the data are plotted as luc/gal, which is representative of NF-κB activity. Each value is the mean (n = 3) with SD < 10%.
Similar articles
- Identification of a novel A20-binding inhibitor of nuclear factor-kappa B activation termed ABIN-2.
Van Huffel S, Delaei F, Heyninck K, De Valck D, Beyaert R. Van Huffel S, et al. J Biol Chem. 2001 Aug 10;276(32):30216-23. doi: 10.1074/jbc.M100048200. Epub 2001 Jun 4. J Biol Chem. 2001. PMID: 11390377 - Epstein-Barr virus-encoded latent membrane protein 1 activates the JNK pathway through its extreme C terminus via a mechanism involving TRADD and TRAF2.
Eliopoulos AG, Blake SM, Floettmann JE, Rowe M, Young LS. Eliopoulos AG, et al. J Virol. 1999 Feb;73(2):1023-35. doi: 10.1128/JVI.73.2.1023-1035.1999. J Virol. 1999. PMID: 9882303 Free PMC article. - The cytokine-inducible zinc finger protein A20 inhibits IL-1-induced NF-kappaB activation at the level of TRAF6.
Heyninck K, Beyaert R. Heyninck K, et al. FEBS Lett. 1999 Jan 15;442(2-3):147-50. doi: 10.1016/s0014-5793(98)01645-7. FEBS Lett. 1999. PMID: 9928991 - ABINs: A20 binding inhibitors of NF-kappa B and apoptosis signaling.
Verstrepen L, Carpentier I, Verhelst K, Beyaert R. Verstrepen L, et al. Biochem Pharmacol. 2009 Jul 15;78(2):105-14. doi: 10.1016/j.bcp.2009.02.009. Epub 2009 Feb 27. Biochem Pharmacol. 2009. PMID: 19464428 Review. - The biology of A20-binding inhibitors of NF-kappaB activation (ABINs).
Verstrepen L, Carpentier I, Beyaert R. Verstrepen L, et al. Adv Exp Med Biol. 2014;809:13-31. doi: 10.1007/978-1-4939-0398-6_2. Adv Exp Med Biol. 2014. PMID: 25302363 Review.
Cited by
- Assembly PCR synthesis of optimally designed, compact, multi-responsive promoters suited to gene therapy application.
Mohamed H, Chernajovsky Y, Gould D. Mohamed H, et al. Sci Rep. 2016 Jul 8;6:29388. doi: 10.1038/srep29388. Sci Rep. 2016. PMID: 27387837 Free PMC article. - Identification of the Key Regulators of Spina Bifida Through Graph-Theoretical Approach.
Tamkeen N, AlOmar SY, Alqahtani SAM, Al-Jurayyan A, Farooqui A, Tazyeen S, Ahmad N, Ishrat R. Tamkeen N, et al. Front Genet. 2021 Apr 6;12:597983. doi: 10.3389/fgene.2021.597983. eCollection 2021. Front Genet. 2021. PMID: 33889172 Free PMC article. - A20: linking a complex regulator of ubiquitylation to immunity and human disease.
Ma A, Malynn BA. Ma A, et al. Nat Rev Immunol. 2012 Nov;12(11):774-85. doi: 10.1038/nri3313. Epub 2012 Oct 12. Nat Rev Immunol. 2012. PMID: 23059429 Free PMC article. Review. - Sepsis-induced suppression of lung innate immunity is mediated by IRAK-M.
Deng JC, Cheng G, Newstead MW, Zeng X, Kobayashi K, Flavell RA, Standiford TJ. Deng JC, et al. J Clin Invest. 2006 Sep;116(9):2532-42. doi: 10.1172/JCI28054. Epub 2006 Aug 17. J Clin Invest. 2006. PMID: 16917541 Free PMC article.
References
- Barnes PJ, Karin M. Nuclear factor-κB: a pivotal transcription factor in inflammatory diseases. N Engl J Med. 1997;336:1066–1071. - PubMed
- Bergmann M, Hart L, Lindsay M, Barnes PJ, Newton R. IκB degradation and nuclear factor-κB DNA binding are insufficient for interleukin-1β and tumor necrosis factor-α induced κB-dependent transcription. J Biol Chem. 1998;273:6607–6610. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous