Transient gene transfer and expression of Smad7 prevents bleomycin-induced lung fibrosis in mice - PubMed (original) (raw)
Transient gene transfer and expression of Smad7 prevents bleomycin-induced lung fibrosis in mice
A Nakao et al. J Clin Invest. 1999 Jul.
Abstract
TGF-beta plays an important role in lung fibrosis, which is a major cause of suffering and death seen in pulmonary disease. Smad7 has been recently identified as an antagonist of TGF-beta signaling. To investigate whether this novel molecule can be exploited for therapy of lung fibrosis, we determined the effect of exogenous Smad7, introduced by a recombinant human type 5 adenovirus vector, on bleomycin-induced lung fibrosis in mice. C57BL/6 mice with bleomycin-induced lungs received an intratracheal injection of a recombinant adenovirus carrying mice Smad7 cDNA. These mice demonstrated suppression of type I precollagen mRNA, reduced hydroxyproline content, and no morphological fibrotic responses in the lungs when compared with mice administered adenovirus carrying Smad6 cDNA. In addition, we found that expression of Smad7 transgene blocked Smad2 phosphorylation induced by bleomycin in mouse lungs. These data indicated that gene transfer of Smad7 (but not Smad6) prevented bleomycin-induced lung fibrosis, suggesting that Smad7 may have applicability in the treatment of pulmonary fibrosis.
Figures
Figure 1
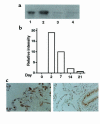
(a) Levels of immunoreactive Smad7 in the lungs of mice after treatment with bleomycin plus AdCMV-Smad7. Expression of exogenous Smad6 or Smad7 derived from an AdCMV-Smad6 or AdCMV-Smad7 construct was detected in the lungs (lane 1: Smad6; lane 2: Smad7), peripheral blood cells (lane 3: Smad7), and spleen (lane 4: Smad7) of bleomycin-treated mice at day 7 by Western blotting using anti-FLAG antibody after infection of AdCMV-Smad6 or AdCMV-Smad7 at day 0. (b) Quantitative analysis of expression of Smad7 transgene in the lungs of mice after treatment with bleomycin plus AdCMV-Smad7. Expression of Smad7 transgene was detected by Western blotting as shown in a, and intensity of the bands of exogenous Smad7 detected at days 0, 2, 7, 14, and 21 was measured using NIH Image software. The relative intensity of the bands at indicated days was expressed as relative intensity compared with that at day 0. (c) Cellular localization of Smad7 transgene in the lungs of mice after treatment with bleomycin plus AdCMV-Smad7. Immunohistochemical staining using anti-FLAG antibody was performed as described in the text. Staining was observed mainly in the nucleus of alveolar epithelial cells and interstitial fibroblast-like cells (left), and bronchial epithelial cells (right).
Figure 2
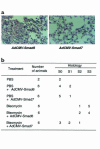
Effect of exogenous Smad7 on bleomycin-induced lung fibrosis in mice. (a) Lung fibrosis was induced by continuous subcutaneous infusion of bleomycin (100 mg/kg for 7 days) from days 0–7 in C57BL/6 mice. Mice were infected with recombinant adenovirus (AdCMV-Smad6 or AdCMV-Smad7) by intratracheal injection with 109 pfu/mouse at day 0. Mice were sacrificed after 4 weeks, and the lungs were fixed in 10% formalin and stained with hematoxylin/eosin/safranin O. Left: treatment with bleomycin plus AdCMV-Smad6. Note the thickened continuous interalveolar septa. Right: treatment with bleomycin plus AdCMV-Smad7. Note the absence of thickened interalveolar septa, although some increased cellularity in interalveolar septa was observed. (b) Scoring of lung fibrosis. Fibrotic changes in mouse lung (S0–S3 = score 0–3, respectively) were graded according to the criteria described in Methods.
Figure 3
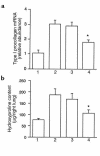
Effect of exogenous Smad7 on bleomycin-induced type I procollagen mRNA expression and hydroxyproline content in mouse lungs. (a) Quantitation of type I procollagen mRNA levels in the lungs removed at day 28. Bar 1: PBS-treated mice; bar 2: mice treated with bleomycin alone; bar 3: mice treated with bleomycin plus AdCMV-Smad6; bar 4: mice treated with bleomycin plus AdCMV-Smad7. Results are expressed as relative abundance of type I procollagen mRNA (type I procollagen mRNA/GAPDH mRNA) and are mean ± SD for 6 mice in each group. *Result is significantly different (P < 0.005) from the mean value of the response (bleomycin alone or bleomycin plus AdCMV-Smad6). (b) Quantitation of hydroxyproline content at day 28 in the right lungs of PBS-treated mice (bar 1), mice treated with bleomycin alone (bar 2), mice treated with bleomycin plus AdCMV-Smad6 (bar 3), and mice treated with bleomycin plus AdCMV-Smad7 (bar 4). Results are mean ± SD for 6 mice in each group. *Result is significantly different (P < 0.001) from the mean value of the response (bleomycin alone or bleomycin plus AdCMV-Smad6).
Figure 4
Effect of exogenous Smad7 on bleomycin-induced pulmonary inflammation and TGF-β production in mouse lungs. (a–c). Leukocyte infiltration into BALF at day 7 of PBS-treated mice (bars 1), mice treated with bleomycin alone (bars 2), mice treated with bleomycin plus AdCMV-Smad6 (bars 3), and mice treated with bleomycin plus AdCMV-Smad7 (bars 4). Results are means ± SD for 6 mice in each group. (d) Levels of TGF-β in BALF at day 7 (open bars) and at day 14 (filled bars) of mice treated with bleomycin alone (bar 1), mice treated with bleomycin plus AdCMV-Smad6 (bar 2), and mice treated with bleomycin plus AdCMV-Smad7 (bar 3). Results are means ± SD for 6 mice in each group.
Figure 5
Effect of exogenous Smad7 expression on bleomycin-induced phosphorylation of Smad2 in the mouse lungs. Levels of immunoreactive phosphorylated Smad2 (a) and Smad2 (b) in the lungs of PBS-treated mice (lane a), mice treated with bleomycin alone (lane b), mice treated with bleomycin plus AdCMV-Smad6 (lane c), and mice treated with bleomycin plus AdCMV-Smad7 (lane d). Lung samples were obtained at day 7 after initial administration of bleomycin. The same filter used in a was reprobed and blotted with Smad2 antibody. Exogenous Smad6 and Smad7 expression at day 7 was confirmed by Western blotting using anti-FLAG antibody as shown in Figure 1a.
Similar articles
- Smad7 but not Smad6 cooperates with oncogenic ras to cause malignant conversion in a mouse model for squamous cell carcinoma.
Liu X, Lee J, Cooley M, Bhogte E, Hartley S, Glick A. Liu X, et al. Cancer Res. 2003 Nov 15;63(22):7760-8. Cancer Res. 2003. PMID: 14633701 - Smad7 and Smad6 differentially modulate transforming growth factor beta -induced inhibition of embryonic lung morphogenesis.
Zhao J, Shi W, Chen H, Warburton D. Zhao J, et al. J Biol Chem. 2000 Aug 4;275(31):23992-7. doi: 10.1074/jbc.M002433200. J Biol Chem. 2000. PMID: 10801843 - Transforming growth factor beta signal transduction in the kidney.
Böttinger EP, Miao J, Schiffer M, Bitzer M, Roberts IS. Böttinger EP, et al. Kidney Blood Press Res. 1998;21(2-4):259-61. doi: 10.1159/000025870. Kidney Blood Press Res. 1998. PMID: 9762849 Review. No abstract available. - Gene therapy and endothelial cell targeting for cancer.
Evans RR, Calmels TP, Pitt BR, Brookens MA, Johnson CS, Modzelewski RA, Lazo JS. Evans RR, et al. Ann N Y Acad Sci. 1994 May 31;716:257-64. doi: 10.1111/j.1749-6632.1994.tb21717.x. Ann N Y Acad Sci. 1994. PMID: 7517652 Review.
Cited by
- Idiopathic pulmonary fibrosis: pathogenesis and therapeutic approaches.
Selman M, Thannickal VJ, Pardo A, Zisman DA, Martinez FJ, Lynch JP 3rd. Selman M, et al. Drugs. 2004;64(4):405-30. doi: 10.2165/00003495-200464040-00005. Drugs. 2004. PMID: 14969575 Review. - A homozygous SFTPA1 mutation drives necroptosis of type II alveolar epithelial cells in patients with idiopathic pulmonary fibrosis.
Takezaki A, Tsukumo SI, Setoguchi Y, Ledford JG, Goto H, Hosomichi K, Uehara H, Nishioka Y, Yasutomo K. Takezaki A, et al. J Exp Med. 2019 Dec 2;216(12):2724-2735. doi: 10.1084/jem.20182351. Epub 2019 Oct 10. J Exp Med. 2019. PMID: 31601679 Free PMC article. - The Genetic and Epigenetic Footprint in Idiopathic Pulmonary Fibrosis and Familial Pulmonary Fibrosis: A State-of-the-Art Review.
Tirelli C, Pesenti C, Miozzo M, Mondoni M, Fontana L, Centanni S. Tirelli C, et al. Diagnostics (Basel). 2022 Dec 9;12(12):3107. doi: 10.3390/diagnostics12123107. Diagnostics (Basel). 2022. PMID: 36553114 Free PMC article. Review. - Targeted AAV5-Smad7 gene therapy inhibits corneal scarring in vivo.
Gupta S, Rodier JT, Sharma A, Giuliano EA, Sinha PR, Hesemann NP, Ghosh A, Mohan RR. Gupta S, et al. PLoS One. 2017 Mar 24;12(3):e0172928. doi: 10.1371/journal.pone.0172928. eCollection 2017. PLoS One. 2017. PMID: 28339457 Free PMC article. - Interleukin-38 overexpression prevents bleomycin-induced mouse pulmonary fibrosis.
Xu Z, Yuan X, Gao Q, Li Y, Li M. Xu Z, et al. Naunyn Schmiedebergs Arch Pharmacol. 2021 Feb;394(2):391-399. doi: 10.1007/s00210-020-01920-3. Epub 2020 Jun 23. Naunyn Schmiedebergs Arch Pharmacol. 2021. PMID: 32577797
References
- Border WA, Noble NA. Transforming growth factor β in tissue fibrosis. N Engl J Med. 1994;10:1286–1292. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases