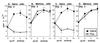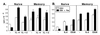Dichotomy between naïve and memory CD4(+) T cell responses to Fas engagement - PubMed (original) (raw)
Dichotomy between naïve and memory CD4(+) T cell responses to Fas engagement
J Desbarats et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Engagement of Fas (APO-1, CD95), a member of the tumor necrosis factor receptor superfamily, can induce apoptotic cell death. However, Fas engagement also can costimulate lymphocyte proliferation. The physiologic regulation of these two outcomes is poorly understood. Here, we have used two systems, the first in vitro and the second in vivo, to demonstrate that naïve and memory CD4(+) T cells display dichotomous responses to Fas ligation. Naïve CD4(+) T cells (CD44(lo), CD45RB+, CD62L+) die as a consequence of Fas ligation in the presence of anti-CD3 antibody, whereas memory T cells (CD44(hi), CD45RB-, CD62L-), freshly isolated from the same starting population and subjected to the same stimulation conditions, are costimulated to proliferate by Fas ligation. In vitro, we demonstrate that CD28-mediated signals or T helper 1 and T helper 2 differentiation cytokines alter the response of naïve T cells, but not of memory T cells, to Fas ligation. In vivo experiments in hen egg lysozyme (HEL) T cell receptor transgenic mice show that CD4(+) T cells from HEL-naïve mice are killed by Fas ligation, but CD4(+) T cells from long-term HEL-exposed mice are costimulated by Fas ligation. Thus, the physiological outcome of Fas ligation in CD4(+) T cells is determined primarily by the antigenic history of the T cell.
Figures
Figure 1
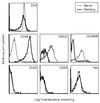
Phenotypes of purified CD4+ naïve and memory cell populations. Naïve (thin line) and memory (thick line) CD4+ cells were prepared from pooled spleens and lymph nodes from B6 mice by negative selection, using T cell purification columns. Naïve cells were purified by removal of CD8+, Ig+, and CD44+ cells. Memory cells were prepared by removal of CD8+, Ig+, and CD62L+ cells.
Figure 2
Fas ligation inhibits naïve CD4+ T cell proliferation and augments memory CD4+ T cell proliferation in B6 (A) but not B6-lpr (B) mice. Naïve (thin lines) and memory (thick lines) CD4+ cell populations were purified as described in Fig. 1 from B6 (A) and B6-lpr (B) pooled peripheral lymphoid cells and stimulated with 1 μg/ml of anti-CD3 antibody and indicated concentrations of anti-Fas antibody for 72 hr. Incorporation of [3H]thymidine was measured as an indicator of proliferation. Engagement of Fas in the absence of anti-CD3 antibody produced no effect.
Figure 3
Fas and CD28 ligation have opposite effects on CD4+ naïve T cell proliferation (A and C) but identical costimulatory effects on CD4+ memory cells (B and D). Purified naïve (A and C) or memory (B and D) CD4+ T cells from B6 mice were cultured with 1 μg/ml of anti-CD3 antibody and indicated concentrations of anti-CD28 (thin line) or anti-Fas (thick line) antibody. Kinetics were determined by harvesting the cells after 48 hr (A and B) or 72 hr (C and D) of stimulation. Incorporation of [3H]thymidine was measured as an indicator of proliferation.
Figure 4
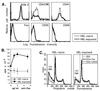
Fas ligation induces apoptotic cell death in naïve but not in memory CD4+ T cells. Naïve (A) or memory (B) cells were stimulated with 1 μg/ml of anti-CD3 antibody alone (dotted line) or with anti-CD3 plus 5 μg/ml of anti-CD28 (solid, thin line) or anti-Fas antibody (thick line) for 48 hr. Cells were permeabilized and stained with DNA-binding solutions and analyzed by flow cytometry. Apoptotic (<2n DNA) cells are those to the left of the gate. For naïve cells (A), 29% of cells were apoptotic (<2n DNA) when stimulated with anti-CD3 alone, 8% were apoptotic when treated with anti-CD3 and anti-CD28 antibodies, and 57% were apoptotic when treated with anti-CD3 and anti-Fas antibodies. For memory cells (B), percentages were 23, 12, and 18% for anti-CD3 alone, anti-CD3 + anti-CD28, and anti-CD3 + anti-Fas treatments, respectively.
Figure 5
The cytokine environment modulates naïve but not memory CD4+ T cell response to Fas ligation. (A) Differentiation cytokines IL-4 (Th2) and IL-12 (Th1) reverse the Fas responsiveness of naïve cells but do not affect the Fas responsiveness of memory cells. Naïve or memory CD4+ T cells were stimulated with 1 μg/ml of anti-CD3 alone (open bars) or 5 μg/ml of anti-Fas antibody (solid bars) in the presence of optimal concentrations of IL-4 or IL-12. Proliferation was assessed by [3H]thymidine incorporation after 48 hr in culture. (B) CD28-mediated costimulation, but not IL-2, reverses the Fas responsiveness of naïve cells. Cells were cultured as in A except that the growth factor IL-2 (10 ng/ml) or anti-CD28 antibody (5 μg/ml) was added instead of differentiation cytokines.
Figure 6
Fas responsiveness of TCR-transgenic CD4+ T cells shifts from Fas-death-sensitive to Fas-costimulated after in vivo exposure to HEL. Purified CD4+ T cells were prepared from cervical and axillary lymph nodes of HEL TCR-transgenic mice that were uninjected (HEL-naïve) or were injected with HEL peptide 24 days previously (HEL-exposed). (A) Cell surface phenotypes of HEL-naïve (thin line) and memory (24 days after HEL exposure; thick line) CD4+ TCR-transgenic T cells were determined by flow cytometry. Anti-Vβ8.2 antibodies recognize the transgenic TCR β chain; 87 and 89% of CD4+ cells were Vβ8.2+ in HEL-naïve and HEL-exposed mice, respectively. Differences in phenotype between memory and naive cells occur only in CD44 and CD45RB (memory markers) expression. (B) Memory cells generated by in vivo exposure to antigen are costimulated by Fas. HEL-naïve (thin line) and memory (thick line) cells were cultured with 1 μg/ml of anti-CD3 antibody plus increasing concentrations of anti-Fas antibody. (C) Fas ligation increases apoptotic death (<2n DNA) in cultures of naïve transgenic cells, but reduces apoptosis in memory cell cultures. CD4+ TCR-transgenic T cells were cultured for 48 hr with anti-CD3 alone (dotted line), anti-CD3 plus anti-CD28 (thin, solid line), or anti-CD3 plus anti-Fas (thick line).
Similar articles
- Responding naive T cells differ in their sensitivity to Fas engagement: early death of many T cells is compensated by costimulation of surviving T cells.
Maksimow M, Santanen M, Jalkanen S, Hänninen A. Maksimow M, et al. Blood. 2003 May 15;101(10):4022-8. doi: 10.1182/blood-2002-06-1904. Epub 2003 Jan 16. Blood. 2003. PMID: 12531803 - TNF receptor 2-deficient CD8 T cells are resistant to Fas/Fas ligand-induced cell death.
Teh HS, Seebaran A, Teh SJ. Teh HS, et al. J Immunol. 2000 Nov 1;165(9):4814-21. doi: 10.4049/jimmunol.165.9.4814. J Immunol. 2000. PMID: 11046004 - Resting and anergic B cells are defective in CD28-dependent costimulation of naive CD4+ T cells.
Ho WY, Cooke MP, Goodnow CC, Davis MM. Ho WY, et al. J Exp Med. 1994 May 1;179(5):1539-49. doi: 10.1084/jem.179.5.1539. J Exp Med. 1994. PMID: 7909325 Free PMC article. - T cell receptor ligation triggers novel nonapoptotic cell death pathways that are Fas-independent or Fas-dependent.
Davidson WF, Haudenschild C, Kwon J, Williams MS. Davidson WF, et al. J Immunol. 2002 Dec 1;169(11):6218-30. doi: 10.4049/jimmunol.169.11.6218. J Immunol. 2002. PMID: 12444127
Cited by
- Differentiation into an Effector Memory Phenotype Potentiates HIV-1 Latency Reversal in CD4+ T Cells.
Kulpa DA, Talla A, Brehm JH, Ribeiro SP, Yuan S, Bebin-Blackwell AG, Miller M, Barnard R, Deeks SG, Hazuda D, Chomont N, Sékaly RP. Kulpa DA, et al. J Virol. 2019 Nov 26;93(24):e00969-19. doi: 10.1128/JVI.00969-19. Print 2019 Dec 15. J Virol. 2019. PMID: 31578289 Free PMC article. - Interleukin 7 up-regulates CD95 protein on CD4+ T cells by affecting mRNA alternative splicing: priming for a synergistic effect on HIV-1 reservoir maintenance.
Yin Y, Zhang S, Luo H, Zhang X, Geng G, Li J, Guo X, Cai W, Li L, Liu C, Zhang H. Yin Y, et al. J Biol Chem. 2015 Jan 2;290(1):35-45. doi: 10.1074/jbc.M114.598631. Epub 2014 Nov 19. J Biol Chem. 2015. PMID: 25411246 Free PMC article. - The NF-κB regulator Bcl-3 governs dendritic cell antigen presentation functions in adaptive immunity.
Tassi I, Claudio E, Wang H, Tang W, Ha HL, Saret S, Ramaswamy M, Siegel R, Siebenlist U. Tassi I, et al. J Immunol. 2014 Nov 1;193(9):4303-11. doi: 10.4049/jimmunol.1401505. Epub 2014 Sep 22. J Immunol. 2014. PMID: 25246497 Free PMC article. - Death receptor-ligand systems in cancer, cell death, and inflammation.
Walczak H. Walczak H. Cold Spring Harb Perspect Biol. 2013 May 1;5(5):a008698. doi: 10.1101/cshperspect.a008698. Cold Spring Harb Perspect Biol. 2013. PMID: 23637280 Free PMC article. Review. - Life in the Fas lane: differential outcomes of Fas signaling.
Brint E, O'Callaghan G, Houston A. Brint E, et al. Cell Mol Life Sci. 2013 Nov;70(21):4085-99. doi: 10.1007/s00018-013-1327-z. Epub 2013 Apr 12. Cell Mol Life Sci. 2013. PMID: 23579628 Free PMC article. Review.
References
- Sprent J, Tough D F. Science. 1994;265:1395–1400. - PubMed
- Sprent J. Cell. 1994;76:315–322. - PubMed
- Sprent J. Curr Opin Immunol. 1997;9:371–379. - PubMed
- Brunner T, Mogil R J, LaFace D, Yoo N J, Mahboubi A, Echeverri F, Martin S J, Force W R, Lynch D H, Ware C F, et al. Nature (London) 1995;373:342–345. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous