Sustained ex vivo expansion of hematopoietic stem cells mediated by thrombopoietin - PubMed (original) (raw)
Sustained ex vivo expansion of hematopoietic stem cells mediated by thrombopoietin
M Yagi et al. Proc Natl Acad Sci U S A. 1999.
Abstract
The hematopoietic stem cell (HSC) is defined as a cell that can either self-replicate or generate daughter cells that are destined to commit to mature cells of different specific lineages. Self-replication of the most primitive HSC produces daughter cells that possess a long (possibly unlimited) clonal lifespan, whereas differentiation of HSC produces daughter cells that demonstrate a progressive reduction of their clonal lifespan, a loss of multilineage potential, and lineage commitment. Previous studies indicated that the proliferation of HSC ex vivo favors differentiation at the expense of self-replication, eventually resulting in a complete loss of HSC. In contrast, transplantation studies have shown that a single HSC can repopulate the marrow of a lethally irradiated mouse, demonstrating that self-renewal of HSC occurs in vivo. Thrombopoietin (TPO) has been shown to function both as a proliferative and differentiative factor for megakaryocytes and as a survival and weakly proliferative factor for HSC. Our studies focused on the effects of exogenous TPO on HSC in mouse long-term bone marrow cultures (LTBMC). Previous results indicate that HSC decline in LTBMC in the absence of TPO. In contrast, the continuous presence of TPO resulted in the generation of both long- and short-term repopulating HSC as detected by an in vivo competitive repopulation assay. HSC were generated over a 4-month period at concentrations similar to normal bone marrow. Our results demonstrate that TPO can mediate the self-replication of HSC in LTBMC, and provide proof that HSC can self-replicate ex vivo.
Figures
Figure 1
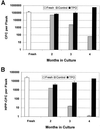
Production of NA cells in LTBMC. Cells harvested from semiweekly feedings of LTBMC were pooled, counted, averaged, and corrected for the volume of media harvested. (A and B) NA cell production by the cultures used for transplants. Two sets of LTBMC are depicted in C.
Figure 2
Production of CFC and HPP-CFC in LTBMC. At various times in culture, 2,000–5,000 NA cells were cultured and colonies were counted as described in Materials and Methods. The frequency of colonies obtained was multiplied by the number of NA cells per flask to obtain the number of CFC per flask. Total number of (A) CFC per flask and (B) HPP-CFC per flask.
Figure 3
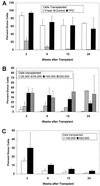
Competitive repopulation assays of culture-derived HSC. (A) Long-term repopulating HSC in 2-month-old TPO-LTBMC, but not control LTBMC. Lethally irradiated C57BL/6 (CD45.2) mice were transplanted with fresh CD45.1 bone marrow cells only, or with 5 × 105 CD45.2 bone marrow cells plus 5.5 × 105 or 1.34 × 106 CD45.1 NA cells from control or TPO-LTBMC. Bars indicate the percent CD45.1+ cells in peripheral blood at various times after transplant. (B) Limiting dilution analysis of cells from 3-month-old TPO-LTBMC. Lethally irradiated C57BL/6 mice were transplanted with 3 × 105 C57BL/6 bone marrow cells, and 2.5 × 104, 5.0 × 104, 1 × 105, or 2 × 105 NA cells from 3-month TPO-LTBMC. (C) HSC in NA cells from 4-month-old TPO-LTBMC. Lethally irradiated animals were transplanted with syngeneic normal bone marrow and 1 × 105 or 5 × 105 NA cells, and analyzed as described above.
Similar articles
- The effect of thrombopoietin on the proliferation and differentiation of murine hematopoietic stem cells.
Sitnicka E, Lin N, Priestley GV, Fox N, Broudy VC, Wolf NS, Kaushansky K. Sitnicka E, et al. Blood. 1996 Jun 15;87(12):4998-5005. Blood. 1996. PMID: 8652812 - Megakaryocytopoiesis in vitro: from the stem cells' perspective.
Cardier JE, Foster DC, Lok S, Jacobsen SE, Murphy MJ Jr. Cardier JE, et al. Stem Cells. 1996;14 Suppl 1:163-72. doi: 10.1002/stem.5530140721. Stem Cells. 1996. PMID: 11012217 - Quantitative assessment of the stem cell self-renewal capacity.
Nakauchi H, Sudo K, Ema H. Nakauchi H, et al. Ann N Y Acad Sci. 2001 Jun;938:18-24; discussion 24-5. doi: 10.1111/j.1749-6632.2001.tb03570.x. Ann N Y Acad Sci. 2001. PMID: 11458506 Review. - Thrombopoietin: biology and clinical potentials.
Miyazaki H, Kato T. Miyazaki H, et al. Int J Hematol. 1999 Dec;70(4):216-25. Int J Hematol. 1999. PMID: 10643146 Review.
Cited by
- JAK2, complemented by a second signal from c-kit or flt-3, triggers extensive self-renewal of primary multipotential hemopoietic cells.
Zhao S, Zoller K, Masuko M, Rojnuckarin P, Yang XO, Parganas E, Kaushansky K, Ihle JN, Papayannopoulou T, Willerford DM, Clackson T, Blau CA. Zhao S, et al. EMBO J. 2002 May 1;21(9):2159-67. doi: 10.1093/emboj/21.9.2159. EMBO J. 2002. PMID: 11980713 Free PMC article. - Thrombopoietin mimetic stimulates bone marrow vascular and stromal niches to mitigate acute radiation syndrome.
Vercellino J, Małachowska B, Kulkarni S, Bell BI, Shajahan S, Shinoda K, Eichenbaum G, Verma AK, Ghosh SP, Yang WL, Frenette PS, Guha C. Vercellino J, et al. Stem Cell Res Ther. 2024 Apr 29;15(1):123. doi: 10.1186/s13287-024-03734-z. Stem Cell Res Ther. 2024. PMID: 38679747 Free PMC article. - HSC Niche Biology and HSC Expansion Ex Vivo.
Kumar S, Geiger H. Kumar S, et al. Trends Mol Med. 2017 Sep;23(9):799-819. doi: 10.1016/j.molmed.2017.07.003. Epub 2017 Aug 8. Trends Mol Med. 2017. PMID: 28801069 Free PMC article. Review. - A robust approach for the generation of functional hematopoietic progenitor cell lines to model leukemic transformation.
Doma E, Mayer IM, Brandstoetter T, Maurer B, Grausenburger R, Menzl I, Zojer M, Hoelbl-Kovacic A, Carlsson L, Heller G, Kollmann K, Sexl V. Doma E, et al. Blood Adv. 2021 Jan 12;5(1):39-53. doi: 10.1182/bloodadvances.2020003022. Blood Adv. 2021. PMID: 33570624 Free PMC article. - c-Mpl-del, a c-Mpl alternative splicing isoform, promotes AMKL progression and chemoresistance.
Li F, Xiong Y, Yang M, Chen P, Zhang J, Wang Q, Xu M, Wang Y, He Z, Zhao X, Huang J, Gu X, Zhang L, Sun R, Sun X, Li J, Ou J, Xu T, Huang X, Cao Y, Xu XR, Karakas D, Li J, Ni H, Zhang Q. Li F, et al. Cell Death Dis. 2022 Oct 13;13(10):869. doi: 10.1038/s41419-022-05315-5. Cell Death Dis. 2022. PMID: 36229456 Free PMC article.
References
- Osawa M, Hanada K, Hamada H, Nakauchi H. Science. 1996;273:242–245. - PubMed
- Dick J E, Magli M C, Huszar D, Phillips R A, Bernstein A. Cell. 1985;42:71–79. - PubMed
- Jordan C T, Lemischka I R. Genes Dev. 1990;4:220–232. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical