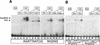The control of trunk Hox specificity and activity by Extradenticle - PubMed (original) (raw)
The control of trunk Hox specificity and activity by Extradenticle
H D Ryoo et al. Genes Dev. 1999.
Abstract
We characterize a 37-bp element (fkh[250]) derived from the fork head (fkh) gene, a natural target of the Hox gene Sex combs reduced (Scr). In vitro, Scr cooperatively binds to this DNA with the Hox cofactor Extradenticle (Exd), and the activation of this enhancer in vivo requires Scr and exd. Other Hox/Exd heterodimers do not activate this element in vivo and do not bind this element with high affinity in vitro. The amino-terminal arm of the Scr homeodomain is crucial for the specific activation of this element in vivo. By mutating two base pairs within this element, we can convert the Scr/Exd-binding site to a Hox/Exd consensus site that binds several different Hox/Exd heterodimers. This element, fkh[250(con)], is activated by Scr, Antennapedia (Antp), and Ultrabithorax (Ubx) but repressed by abdominal-A (abd-A). We also show that Scr and Exd are only able to activate the fkh[250] element during the early stages of embryogenesis because, by stage 11, Scr negatively regulates the gene homothorax (hth), which is required for the nuclear localization of Exd. These results suggest that Exd is a specificity cofactor for the trunk Hox genes, and that the control of Exd subcellular localization is a mechanism to regulate Hox activity during development.
Figures
Figure 1
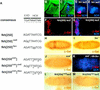
The fkh[250] element includes a potential Hox/Exd-binding site and requires Scr and exd for its activation in embryos. (A) The sequence of the Hox/Exd-binding sites within fkh[250] and fkh[250con] are compared with the consensus-binding site. The two differences between fkh[250] and fkh[250con] (basepairs 6 and 9) are underlined, whereas mutations in the Exd or Hox half-sites are indicated with lowercase letters. (B,C) Fluorescent images of a stage-10 embryo (lateral view of PS 2 region) stained for Exd (green) and Scr (blue). (D,E) Fluorescent images of a stage-10 fkh–lacZ embryo (lateral view of PS 2 region) stained for β-gal (red) and Scr (blue). At this stage, Exd is nuclear in all Scr+ cells, and a subset of Scr+ cells begin to express fkh–lacZ. (F,G) Fluorescent images of a wild-type fkh[250]–lacZ embryo stained for β-gal (red) and Scr (blue). Only a subset of the Scr+ cells express fkh[250]–lacZ. (H,I,J) Wild-type (H), _Scr_− (I), and _exd_mat−,zyg− (J) fkh[250]–lacZ embryos histochemically stained for β-gal (brown). PS 2 expression is absent in the _Scr_− embryo but the lateral staining remains. All detectable reporter gene expression is absent in the _exd_mat−,zyg− embryo. (K) Fluorescent image of an _exd_mat−,zyg−; fkh[250]–lacZ em-bryo in which Scr was ubiquitously ex-pressed, and stained for β-gal (red) and Scr (blue). Ectopic Scr was not able to activate fkh[250]–lacZ in these embryos. (L,M) fkh[250]exd–lacZ (L) or fkh[250]Scr–lacZ (M) embryos histochemically stained for β-gal (brown). Mutation of the Exd half-site (L) or Scr half-site (M) abolishes PS 2 expression. (F_–_M) Ventral views of ∼stage 10 embryos, with anterior to the left.
Figure 2
Effects of ectopic Hox expression on the fkh[250] element. (A) Structure of Antp, Scr, and chimeric proteins, G26 and K26. The YPWM and homeodomain are shown as small and large boxes, respectively. Antp-specific sequences are shown in light gray, Scr-specific sequences are shown in dark gray, and homeodomain sequences that are identical in both proteins are shown in white. The only amino acid differences between G26 and K26 are in the amino-terminal arm of the homeodomain, as indicated. (B_–_G) All embryos are ventral views histochemically stained for β-gal (black). Wild-type fkh[250]–lacZ expression is limited to PS 2 (B); but on ectopic Scr expression, β-gal staining is expanded anteriorly and posteriorly (C, arrows). Ectopic Antp (D) or Ubx (E) do not activate this element. Ectopic expression of G26 expands β-gal (F, arrows), whereas ectopic K26 does not (G).
Figure 2
Effects of ectopic Hox expression on the fkh[250] element. (A) Structure of Antp, Scr, and chimeric proteins, G26 and K26. The YPWM and homeodomain are shown as small and large boxes, respectively. Antp-specific sequences are shown in light gray, Scr-specific sequences are shown in dark gray, and homeodomain sequences that are identical in both proteins are shown in white. The only amino acid differences between G26 and K26 are in the amino-terminal arm of the homeodomain, as indicated. (B_–_G) All embryos are ventral views histochemically stained for β-gal (black). Wild-type fkh[250]–lacZ expression is limited to PS 2 (B); but on ectopic Scr expression, β-gal staining is expanded anteriorly and posteriorly (C, arrows). Ectopic Antp (D) or Ubx (E) do not activate this element. Ectopic expression of G26 expands β-gal (F, arrows), whereas ectopic K26 does not (G).
Figure 3
Hox/Exd-binding preferences of fkh[250] and fkh[250con]. (A) EMSA using the fkh[250] oligo with Hox and Exd proteins as indicated. There is a preference for binding Scr/Exd heterodimers over Antp/Exd, Ubx/Exd, and Abd-A/Exd heterodimers. (B) EMSA with the fkh[250]exd or fkh[250]Scr oligos with Scr and Exd as indicated. Mutation of the Exd half-site (fkh[250]exd) or the Scr half-site (fkh[250]Scr) abolished Scr/Exd complex formation. (C) EMSA with the fkh[250con] oligo with Hox and Exd proteins as indicated. All four heterodimers (Scr/Exd, Antp/Exd, Ubx/Exd, Abd-A/Exd) bound strongly to the fkh[250con] oligonucleotide. The concentrations of all proteins used in C were identical to those in A, and these EMSAs were carried out at the same time.
Figure 3
Hox/Exd-binding preferences of fkh[250] and fkh[250con]. (A) EMSA using the fkh[250] oligo with Hox and Exd proteins as indicated. There is a preference for binding Scr/Exd heterodimers over Antp/Exd, Ubx/Exd, and Abd-A/Exd heterodimers. (B) EMSA with the fkh[250]exd or fkh[250]Scr oligos with Scr and Exd as indicated. Mutation of the Exd half-site (fkh[250]exd) or the Scr half-site (fkh[250]Scr) abolished Scr/Exd complex formation. (C) EMSA with the fkh[250con] oligo with Hox and Exd proteins as indicated. All four heterodimers (Scr/Exd, Antp/Exd, Ubx/Exd, Abd-A/Exd) bound strongly to the fkh[250con] oligonucleotide. The concentrations of all proteins used in C were identical to those in A, and these EMSAs were carried out at the same time.
Figure 4
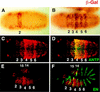
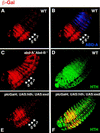
Different expression patterns dictated by fkh[250]–lacZ and fkh[250con]–lacZ in vivo. (A,B) Histochemically stained fkh[250]–lacZ (A) and fkh[250con]–lacZ (B) embryos stained for β-gal (brown). Expression of the fkh[250]–lacZ element is confined to PS 2 (A) and expression of fkh[250con]–lacZ is observed from PS 2 through PS 6 (B). (C_–_F) Fluorescent double labeling of fkh[250con]–lacZ embyros for β-gal (red) and Antp (green) (C,D), or En (green) (E,F) localizes the fkh[250con]–lacZ expression domain from PS 2 to PS 6. Expression of fkh[250con]–lacZ in PS 14 and PS 15 can be seen in (E and F).
Figure 5
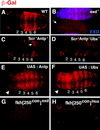
fkh[250con]–lacZ expression requires Scr, Antp, Ubx, and exd and both halves of the Hox/Exd-binding site. (A_–_F) fkh[250con]–lacZ expression in (A) wild type, (B) _exd_mat−,zyg−, (C) _Scr_− _Antp_−, (D) _Scr_− _Antp_− _Ubx_−, (E) ectopic Antp, and (F) ectopic Ubx backgrounds. All embryos were stained for β-gal (red). In B the embryos were costained for Exd to identify the _exd_mat−,zyg− embryos (arrow); the embryo that stains in B is paternally rescued for exd (mat−, zyg+). In _Scr_− _Antp_− embryos (C), expression in PS 2 to PS 5 is absent but remains in PS 6 (arrow). In _Scr_− _Antp_− _Ubx_− embryos (D), expression is absent in PS 2 to PS 6. The arrowheads in E and F point to ectopic β-gal expression in the head induced by Antp or Ubx. (G,H) fkh[250con]exd–lacZ (G) or fkh[250con]Scr–lacZ (H) embryos stained for β-gal (red). Mutation of the Exd half-site (G), or the Hox half-site (F) abolishes fkh[250con]–lacZ expression in PS 2 to PS 6. See Fig. 1A for the sequences of the mutant-binding sites.
Figure 6
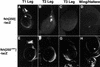
Expression of fkh[250]–lacZ and fkh[250con]–lacZ in imaginal discs. (A_–_D) Fluorescent images of imaginal discs from fkh[250]–lacZ larvae stained for β-gal (white). fkh[250]–lacZ is expressed in the T1 leg disc (A), but not in the T2 (B), or T3 (C) leg discs, and not in the wing (D) or haltere (not shown) discs. The cells staining in B are mesodermal cells associated with the T2 leg disc that express Scr (Percival-Smith et al. 1998). (E_–_H) Fluorescent images of imaginal discs from fkh[250con]–lacZ larvae stained for β-gal (white). fkh[250con]–lacZ is expressed in all thoracic discs, including T1 (E), T2 (F), and T3 (G) leg discs, wing (w) and haltere (h) discs (H).
Figure 7
abd-A represses fkh[250con]–lacZ in abdominal segments. (A,B) Fluorescent images of a wild-type fkh[250con]–lacZ embryo double stained for Abd-A (blue) and β-gal (red). β-Gal expression is weak in the abd-A domain. (C) Fluorescent image of a fkh[250con]–lacZ; _abd-A_− _Abd-B_− embryo stained for β-gal (red). Derepression of β-gal in the abd-A domain is observed. (D) Fluorescent image of a wild-type embryo stained for Hth (green), which is weak in the abd-A domain. (E,F) Fluorescent image of a ptc–Gal4; UAS:hth; UAS:exd embryo stained for Hth (green) and β-gal (red). Despite ectopic Hth and nuclear Exd (not shown), fkh[250con]–lacZ is still repressed in the abd-A domain.
Figure 8
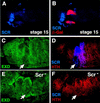
Scr represses hth, resulting in the cytoplasmic localization of Exd in PS 2 of stage 12 and older embryos. (A,B) Lateral view of a stage 15 fkh–lacZ embryo doubly stained for β-gal (red) and Scr (blue). At this stage, the expression of fkh–lacZ is limited to the salivary glands and shows no overlap with Scr. (C,D) In stage 12 embryos, PS 2 cells express Scr (blue), have cytoplasmic Exd (green) and no Hth (red) (arrows). (E,F) In _Scr_− stage 12 embryos, Exd is nuclear (E), and Hth is expressed in this region of the embryo (arrows).
Similar articles
- Regulation of Hox target genes by a DNA bound Homothorax/Hox/Extradenticle complex.
Ryoo HD, Marty T, Casares F, Affolter M, Mann RS. Ryoo HD, et al. Development. 1999 Nov;126(22):5137-48. doi: 10.1242/dev.126.22.5137. Development. 1999. PMID: 10529430 - Hox repression of a target gene: extradenticle-independent, additive action through multiple monomer binding sites.
Galant R, Walsh CM, Carroll SB. Galant R, et al. Development. 2002 Jul;129(13):3115-26. doi: 10.1242/dev.129.13.3115. Development. 2002. PMID: 12070087 - Tarsus determination in Drosophila melanogaster.
Percival-Smith A, Teft WA, Barta JL. Percival-Smith A, et al. Genome. 2005 Aug;48(4):712-21. doi: 10.1139/g05-021. Genome. 2005. PMID: 16094438 - Hox genes: from master genes to micromanagers.
Akam M. Akam M. Curr Biol. 1998 Sep 24;8(19):R676-8. doi: 10.1016/s0960-9822(98)70433-6. Curr Biol. 1998. PMID: 9768351 Review. - Hox codes and positional specification in vertebrate embryonic axes.
Hunt P, Krumlauf R. Hunt P, et al. Annu Rev Cell Biol. 1992;8:227-56. doi: 10.1146/annurev.cb.08.110192.001303. Annu Rev Cell Biol. 1992. PMID: 1362074 Review.
Cited by
- Cofactor binding evokes latent differences in DNA binding specificity between Hox proteins.
Slattery M, Riley T, Liu P, Abe N, Gomez-Alcala P, Dror I, Zhou T, Rohs R, Honig B, Bussemaker HJ, Mann RS. Slattery M, et al. Cell. 2011 Dec 9;147(6):1270-82. doi: 10.1016/j.cell.2011.10.053. Cell. 2011. PMID: 22153072 Free PMC article. - To Be Specific or Not: The Critical Relationship Between Hox And TALE Proteins.
Merabet S, Mann RS. Merabet S, et al. Trends Genet. 2016 Jun;32(6):334-347. doi: 10.1016/j.tig.2016.03.004. Epub 2016 Apr 8. Trends Genet. 2016. PMID: 27066866 Free PMC article. Review. - Organ-specific gene expression: the bHLH protein Sage provides tissue specificity to Drosophila FoxA.
Fox RM, Vaishnavi A, Maruyama R, Andrew DJ. Fox RM, et al. Development. 2013 May;140(10):2160-71. doi: 10.1242/dev.092924. Epub 2013 Apr 11. Development. 2013. PMID: 23578928 Free PMC article. - In vivo Hox binding specificity revealed by systematic changes to a single cis regulatory module.
Sánchez-Higueras C, Rastogi C, Voutev R, Bussemaker HJ, Mann RS, Hombría JC. Sánchez-Higueras C, et al. Nat Commun. 2019 Aug 9;10(1):3597. doi: 10.1038/s41467-019-11416-1. Nat Commun. 2019. PMID: 31399572 Free PMC article. - Alternative splicing modulates Ubx protein function in Drosophila melanogaster.
Reed HC, Hoare T, Thomsen S, Weaver TA, White RA, Akam M, Alonso CR. Reed HC, et al. Genetics. 2010 Mar;184(3):745-58. doi: 10.1534/genetics.109.112086. Epub 2009 Dec 28. Genetics. 2010. PMID: 20038634 Free PMC article.
References
- Abu-Shaar M, Mann RS. Generation of multiple antagonistic domains along the proximodistal axis during Drosophila leg development. Development. 1998;125:3821–3830. - PubMed
- Andrew DJ. Regulation and formation of the Drosophila salivary glands. Ann NY Acad Sci. 1998;15:55–69. - PubMed
- Aspland SE, White RA. Nucleocytoplasmic localisation of extradenticle protein is spatially regulated throughout development in Drosophila. Development. 1997;124:741–747. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases