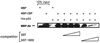The human papillomavirus type 16 E6 oncoprotein can down-regulate p53 activity by targeting the transcriptional coactivator CBP/p300 - PubMed (original) (raw)
The human papillomavirus type 16 E6 oncoprotein can down-regulate p53 activity by targeting the transcriptional coactivator CBP/p300
H Zimmermann et al. J Virol. 1999 Aug.
Abstract
The transforming proteins of the small DNA tumor viruses, simian virus 40 (SV40), adenovirus, and human papillomavirus (HPV) target a number of identical cellular regulators whose functional abrogation is required for transformation. However, while both adenovirus E1A and SV40 large T transforming properties also depend on the targeting of the transcriptional coactivator CBP/p300, no such interaction has been described for the HPV oncoprotein E6 or E7. Here, we demonstrate that the HPV-16 E6 protein, previously shown to facilitate the degradation of p53 in a complex with E6-associated protein (E6AP), also targets CBP/p300 in an interaction involving the C-terminal zinc finger of E6 and CBP residues 1808 to 1826. Furthermore, this interaction is limited to E6 proteins of high-risk HPVs associated with cervical cancer that have the capacity to repress p53-dependent transcription. An HPV-16 E6 mutant (L50G) that binds CBP/p300, but not E6AP, is still capable of down-regulating p53 transcriptional activity. Thus, HPV E6 proteins possess two distinct mechanisms by which to abrogate p53 function: the repression of p53 transcriptional activity by targeting the p53 coactivator CBP/p300, and the removal of cellular p53 protein through the proteosome degradation pathway.
Figures
FIG. 1
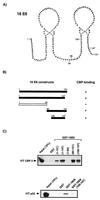
16E6 interacts with the transcriptional coactivator CBP/p300. (A) Equal amounts of partially purified full-length (FL) CBP/p300 from HeLa nuclear extract were passed over GST, GST-16E6, GST-P/CAF, and GST-YY1 micro-affinity columns. After SDS-PAGE and transfer to PVDF membranes, Western blot analysis detected the presence of CBP/p300. The positions of the molecular weight markers are also indicated. (B) GST micro-affinity columns were used to detect the interaction of IVT radiolabelled 16E6 with GST-CBP II (residues 1621 to 1877). No interaction was detected for the control GST column or the GST-CBP I (residues 461 to 662) column. The lower panel shows the Coomassie blue-stained SDS-gel of GST and GST fusion proteins eluted from the micro-affinity columns and also shows the molecular weight marker ladder. (C) Comparison of the 16E6-CBP II interaction with known E1A-CBP II and 16E6-E6AP interactions in GST micro-affinity column assays. (D) Demonstration of a direct interaction between 16E6 and CBP using two recombinant bacterially expressed proteins. GST or GST-E6 was passed over a column containing MBP-CBP (residues 1808 to 1852) fusion protein. Bound GST-fusion protein was detected by Western blot analysis using an anti-GST antibody. The MBP-CBP fusion protein was also passed over a GST or GST-E6 column, and the interaction detected with an anti-MBP antibody.
FIG. 2
Identification of an HPV-16 E6 binding site on CBP/p300. (A) GST-CBP fusion constructs used in micro-affinity column experiments to define CBP sequences capable of binding 16E6 are shown schematically. A 19-amino-acid sequence of CBP (residues 1808 to 1826; lane 7) and larger fragments containing this sequence are able to bind 16E6. Deletion into these sequences abolishes E6 binding (lane 8). Also shown is an alignment of this 19-residue binding site of CBP and the corresponding p300 sequence. Eighteen asterisks represent the conservation of 18-amino-acid residues in that sequence, while + represents the single conservative change. (B) 16E6 and Ad E1A bind the same 19-amino-acid motif in CBP. The interaction between 16E6 and GST-CBP (1765 to 1852) can be disrupted by the presence of a peptide (pep) consisting of Ad E1A sequences previously shown to bind the CBP TRAM (57a). This observation is specific because a peptide containing mutant Ad E1A sequences fails to prevent the E6-CBP interaction. WT, wild type; Mut, mutant.
FIG. 3
Mapping of a 16E6 region involved in the interaction with CBP. (A) Amino acid sequence of the 16E6 protein. The two zinc finger structures are hypothetical, since they have not been based on spectroscopic methods but are supported by the strict conservation of eight cysteine residues in all HPV E6 proteins, as well as by the stoichiometric binding of zinc ions by E6 molecules (7, 21). Indicated are the numbers of the amino acid residues which mark the start or end points of 16E6 fragments used in interaction studies. (B) Schematic representation of GST-E6 fusion constructs used in micro-affinity column assays. (C) Interaction experiments define a region between 16E6 residues 100 to 147 as sufficient for the binding of CBP. This same region is not able to bind p53, however, indicating that p53 and CBP binding are dependent on different 16E6 domains.
FIG. 4
The E6-CBP/p300 interaction is specific for E6 proteins of high-risk HPVs. (A) Micro-affinity column experiments using either GST fusion or IVT E6 proteins demonstrate that only E6 proteins from the high-risk HPV types 16 and 18, but not the low-risk HPV types 6 and 11, are capable of interacting with CBP. Replacing the 16E6 sequences (residues 107 to 135) that have been implicated in CBP binding with those from 11E6 results in a chimeric protein (GST-16/11) that can no longer bind CBP. (B) Mammalian two-hybrid experiments (described in Materials and Methods), shown schematically, indicate that the distinction between E6 proteins of high-risk and low-risk HPVs extends to the in vivo interaction with the CBP II domain. Activation of the G5E1BCAT reporter is seen only after cotransfection of GAL4-16E6 and CBP II-VP16, not for those experiments in which GAL4-11E6 or CBP I-VP16 proteins were expressed.
FIG. 5
The 16E6 mutant L50G binds CBP but is unable to interact with E6AP or p53 and cannot degrade p53 in vitro or in vivo. (A) Schematic representation of the 16E6 mutant (mut) L50G showing the position of the amino acid exchange in the first zinc finger (marked by +) and the identified CBP interaction (binding [bdg.]) domain within the second zinc finger (bold line). GST micro-affinity column experiments using IVT 16E6 L50G protein demonstrate the ability of this mutant to interact with GST-CBP. (B) Similar in vitro micro-affinity column experiments show that unlike the wild-type 16E6 protein but similar to 11E6, the 16E6 mutant L50G is unable to interact with either GST-E6AP or GST-p53. The lower panels indicate the loading of GST, GST-E6AP, and GST-p53 proteins on the micro-affinity columns. (C) The upper panel shows p53 degradation assays using IVT 35S-labelled p53 mixed with various IVT E6 proteins. The numbered columns indicate the levels of p53 protein after various incubation times (0, 30, 90, and 180 min) at room temperature. The lower panel shows an in vivo degradation assay in which cellular levels of p53 are detected by Western blot analysis after cotransfection of U2-OS cells with p53 and 16E6 constructs. The numbers represent the incubation period (in minutes) in media containing cycloheximide before harvesting of the cellular proteins (see Materials and Methods). While the transfection of wild-type 16E6 leads to an observed decrease in cellular p53 levels after 60 min, the 16E6 mutant L50G is abrogated in this capacity.
FIG. 6
16E6 protein can disrupt a p53-CBP interaction in vitro. Bacterially expressed His-p53 can interact directly with bacterially expressed MBP-CBP II. While this interaction is not disrupted by the presence of purified GST protein, it is disrupted by increasing amounts of purified GST-16E6 in a concentration-dependent manner. MBP-Ab, antibody specific to MBP.
FIG. 7
HPV-16 E6 targets the ability of CBP to activate p53-dependent transcription. (A) U2-OS cells were transfected with the p53-responsive CAT reporter (PG13) or a control vector with mutated p53 binding sites (MG15). Cotransfection of expression vectors for viral proteins show that HPV proteins able to interact with CBP can down-regulate p53 transactivation to a level comparable with Ad E1A. Those that fail to interact with CBP also fail to down-regulate p53-dependent transcription. (B) Overexpression of full-length CBP in experiments similar to those described above show that HPV proteins able to interact with CBP, including the HPV-16 L50G mutant, abolish the CBP-dependent superactivation of p53-dependent transcription seen with full-length CBP alone.
Similar articles
- E6 oncoprotein represses p53-dependent gene activation via inhibition of protein acetylation independently of inducing p53 degradation.
Thomas MC, Chiang CM. Thomas MC, et al. Mol Cell. 2005 Jan 21;17(2):251-64. doi: 10.1016/j.molcel.2004.12.016. Mol Cell. 2005. PMID: 15664194 - Complementation of a p300/CBP defective-binding mutant of adenovirus E1a by human papillomavirus E6 proteins.
Bernat A, Massimi P, Banks L. Bernat A, et al. J Gen Virol. 2002 Apr;83(Pt 4):829-833. doi: 10.1099/0022-1317-83-4-829. J Gen Virol. 2002. PMID: 11907332 - Down regulation of the interleukin-8 promoter by human papillomavirus type 16 E6 and E7 through effects on CREB binding protein/p300 and P/CAF.
Huang SM, McCance DJ. Huang SM, et al. J Virol. 2002 Sep;76(17):8710-21. doi: 10.1128/jvi.76.17.8710-8721.2002. J Virol. 2002. PMID: 12163591 Free PMC article. - Cellular targets of the oncoproteins encoded by the cancer associated human papillomaviruses.
Howley PM, Münger K, Romanczuk H, Scheffner M, Huibregtse JM. Howley PM, et al. Princess Takamatsu Symp. 1991;22:239-48. Princess Takamatsu Symp. 1991. PMID: 1668886 Review. - The role of TP53 in Cervical carcinogenesis.
Tommasino M, Accardi R, Caldeira S, Dong W, Malanchi I, Smet A, Zehbe I. Tommasino M, et al. Hum Mutat. 2003 Mar;21(3):307-12. doi: 10.1002/humu.10178. Hum Mutat. 2003. PMID: 12619117 Review.
Cited by
- In Silico Approach for SAR Analysis of the Predicted Model of DEPDC1B: A Novel Target for Oral Cancer.
Ahuja P, Singh K. Ahuja P, et al. Adv Bioinformatics. 2016;2016:3136024. doi: 10.1155/2016/3136024. Epub 2016 Feb 29. Adv Bioinformatics. 2016. PMID: 27034663 Free PMC article. - Transcriptome and microbiome-immune changes across preinvasive and invasive anal cancer lesions.
Lacunza E, Fink V, Salas ME, Gun AM, Basiletti JA, Picconi MA, Golubicki M, Robbio J, Kujaruk M, Iseas S, Williams S, Figueroa MI, Coso O, Cahn P, Ramos JC, Abba MC. Lacunza E, et al. JCI Insight. 2024 Jul 18;9(16):e180907. doi: 10.1172/jci.insight.180907. JCI Insight. 2024. PMID: 39024554 Free PMC article. - The Molecular Interplay between Human Oncoviruses and Telomerase in Cancer Development.
Tornesello ML, Cerasuolo A, Starita N, Tornesello AL, Bonelli P, Tuccillo FM, Buonaguro L, Isaguliants MG, Buonaguro FM. Tornesello ML, et al. Cancers (Basel). 2022 Oct 26;14(21):5257. doi: 10.3390/cancers14215257. Cancers (Basel). 2022. PMID: 36358677 Free PMC article. Review. - Papillomavirus E6 oncoproteins.
Vande Pol SB, Klingelhutz AJ. Vande Pol SB, et al. Virology. 2013 Oct;445(1-2):115-37. doi: 10.1016/j.virol.2013.04.026. Epub 2013 May 24. Virology. 2013. PMID: 23711382 Free PMC article. Review. - Pitx2a binds to human papillomavirus type 18 E6 protein and inhibits E6-mediated P53 degradation in HeLa cells.
Wei Q. Wei Q. J Biol Chem. 2005 Nov 11;280(45):37790-7. doi: 10.1074/jbc.M502974200. Epub 2005 Aug 29. J Biol Chem. 2005. PMID: 16129685 Free PMC article.
References
- Ait-Si-Ali S, Ramirez S, Barre F X, Dkhissi F, Magnaghi-Jaulin L, Girault J A, Robin P, Knibiehler M, Pritchard L L, Ducommun B, Trouche D, Harel-Bellan A. Histone acetyltransferase activity of CBP is controlled by cycle-dependent kinases and oncoprotein E1A. Nature. 1998;396:184–186. - PubMed
- Arany Z, Newsome D, Oldread E, Livingston D M, Eckner R. A family of transcriptional adapter proteins targeted by the E1A oncoprotein. Nature. 1995;374:81–84. - PubMed
- Avantaggiati M L, Ogryzko V, Gardner K, Giordano A, Levine A S, Kelly K. Recruitment of p300/CBP in p53-dependent signal pathways. Cell. 1997;89:1175–1184. - PubMed
- Bagchi S, Raychaudhuri P, Nevins J R. Adenovirus E1A proteins can dissociate heteromeric complexes involving the E2F transcription factor: a novel mechanism for E1A trans-activation. Cell. 1990;62:659–669. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous